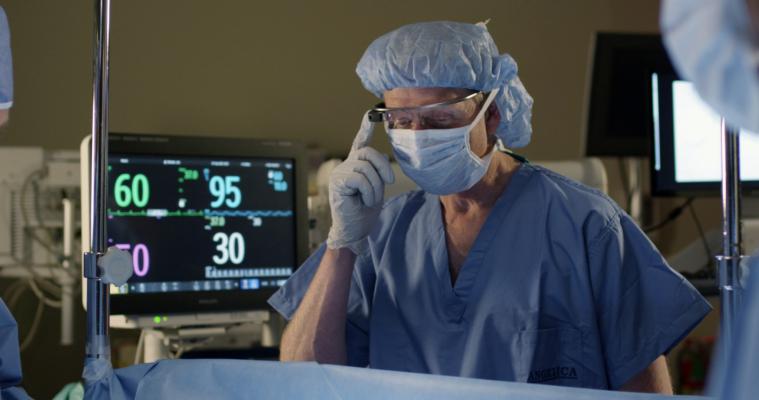
October 7, 2013 — Royal Philips and Accenture announced the creation of a proof-of-concept (POC) demonstration that uses a Google Glass head-mounted display for researching ways to improve the effectiveness and efficiency of performing surgical procedures. The demonstration connects Google Glass to Philips IntelliVue Solutions and proves the concept of seamless transfer of patient vital signs into Google Glass, potentially providing physicians with hands-free access to critical clinical information.
The new concept demonstration depicts how a doctor wearing the display could simultaneously monitor a patient’s vital signs and react to surgical procedural developments without having to turn away from the patient or procedure. A physician could also monitor a patient’s vital signs remotely or enlist assistance from doctors in other locations.
“We live in a world where being nimble is key and industry-altering ideas need to be converted to practical solutions that people can use,” said Michael Mancuso, CEO, patient care and clinical informatics at Philips Healthcare. “This research explores how doctors can achieve better access to the right information at the right time so they can focus on more efficient and effective patient care. It’s a first step in researching how existing technologies can be applied to improve the quality of life of patients.”
Researchers from Philips’ newly created Digital Accelerator Lab, a cross-sector innovation platform with labs based in the Netherlands and India, collaborated with researchers from Accenture Technology Labs to explore the potential use of Google Glass in clinical settings. The goal was to create the first proof of concept for Google Glass and Philips IntelliVue Solutions and then to begin exploring additional opportunities to integrate Google Glass seamlessly with Philips healthcare solutions.
“Accenture’s work with Philips showcases a powerful use of wearable devices in the healthcare industry, helping physicians perform their jobs more effectively and enhancing care for patients,” said Paul Daugherty, chief technology officer, Accenture. “This exciting work highlights the potential of digital technologies to transform the way we work and live, and we’re pleased to have collaborated with Philips to help bring this vision to life.”
Aside from the possibility of operating in a hands-free environment, the Google Glass IntelliVue Solution research effort was developed to explore how to enhance a clinician’s mobility by allowing the seamless transfer of patient information while on the go. Further research may indicate how to possibly enable clinicians to keep their focus on the patient while simultaneously obtaining a live view of critical patient monitoring data.
Additional topics for research may include:
• Accessing a near real-time feed of vital signs in Google Glass
• Calling up images and other patient data by clinicians from anywhere in the hospital
• Accessing a pre-surgery safety checklist
• Giving clinicians the ability to view the patient in the recovery room after surgery
• Conducting live, first-person point-of-view videoconferences with other surgeons or medical personnel
• Recording surgeries from a first-person point-of-view for training purposes
For more information: www.philips.com, www.accenture.com


 January 15, 2026
January 15, 2026 









