October 13, 2023 — InfoBionic, Inc. today announced that it received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for MoMe ARC, their 3rd Generation remote ECG monitoring device paired with their inaugural Bluetooth diagnostic 6-lead sensor designed to aid physicians in their diagnosis of cardiac arrhythmias in patients with a demonstrated need for cardiac monitoring. “We’re thrilled to announce FDA 510(k) clearance to market the MoMe ARC solution, which supports our mission to create superior patient monitoring solutions for arrhythmia detection and virtual care and chronic disease management,” said Dave MacCutcheon, Regulatory and Chief Operating Officer at InfoBionic.
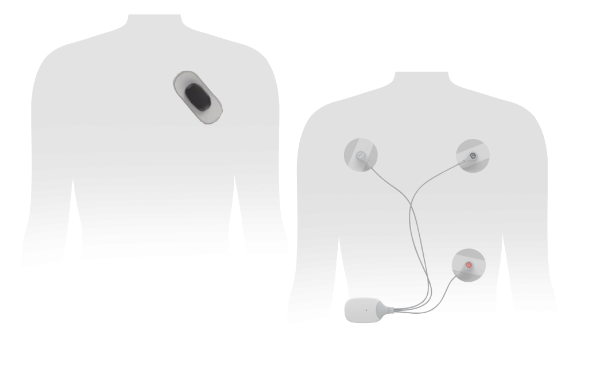
MacCutcheon further points out that “MoMe ARC is a solution that includes a 4-in-1 Gateway device that seamlessly transitions between 2-day in addition to Extended Holter tests, Event and MCT modes remotely, streamlining patient monitoring time and minimizing delays. In addition, MoMe ARC leverages a comprehensive cloud-based proprietary platform to deliver on-demand, actionable data and analytics directly to the clinicians. Further it incorporates our initial sleek body worn Sensor in a new lightweight form factor which is decoupled from the device Gateway communicating through the latest implementations of Bluetooth connectivity and ushers in a new era of wearability convenience yet maintains the ECG quality of a multi-lead tracing thus bringing convenience and quality together for the first time. The MoMe ARC is designed so patients can wear it discretely and comfortably during monitoring using standard electrodes. The Sensor is paired to the ARC Gateway which leverages a cellular connection to the MoMe Software Platform empowering physicians to transform the efficiency with which they manage cardiac arrhythmia detection and monitoring processes for their patients.”
This next generation device builds on the market success of innovative MoMe Kardia II by providing a decoupled 2-channel – 6-Lead Sensor. Added foundational technologies make the device capable of connecting to other Bluetooth enabled health monitoring devices. K230265 is cleared for use under Product Code DSI - Arrhythmia Detector and Alarm (Including ST-Segment Measurement and Alarm). The ECG data is transmitted in near-real time and analyzed by the MoMe software platform via a suite of robust server-based algorithms; and when indicated, data identified by these algorithms is flagged for clinician review. MoMe ARC requires no patient intervention to capture or analyze data, however it does provide a patient event trigger and symptom description selection through a new screen similar to that of a smart watch.
InfoBionic expects to begin shipping the new generation MoMe ARC Device in Q4 2023.
For more information: www.infobionic.com


 January 15, 2026
January 15, 2026 









