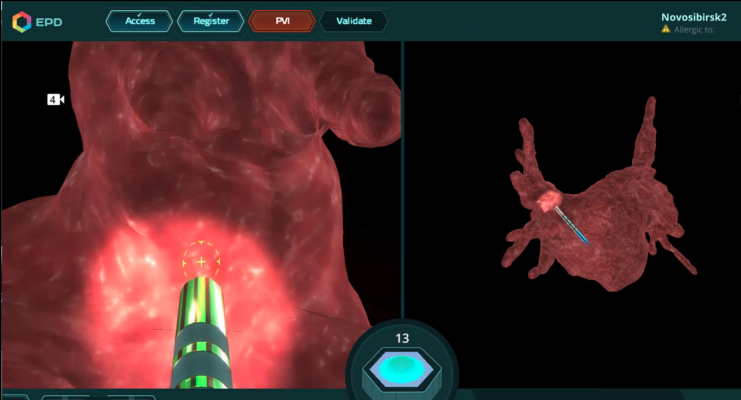
June 5, 2018 — Philips Healthcare has signed an agreement to acquire EPD Solutions, a provider of image-guidance in catheter ablation procedures for cardiac arrhythmias, for $292 million Euros. EPD’s cardiac imaging and navigation system helps electrophysiologists (EPs) navigate the heart by generating a detailed 3-D image of the cardiac anatomy, while also pinpointing the location and orientation of catheters during the diagnostic and therapeutic procedures for cardiac arrhythmias. This new technology has the potential to simplify navigation and treatment, immediately assess the treatment result and ultimately enhance procedure efficacy.
Watch a video showing how this technology helps guide EP ablation procedures.
Watch a video showing how the system illustrated ablation points and can show gaps between lesions.
The acquisition will complement Philips’ portfolio of interventional imaging systems, smart catheters, planning and navigation software and services, and will allow the company to introduce new solutions in the 2 billion Euro market for image-guided treatment of cardiac arrhythmias, which is growing at a double-digit rate, according to Philips. The company will acquire EPD for an upfront cash consideration of 250 million Euros and deferred, milestone dependent payments. In connection with these contingent payments, the company expects to recognize a provision of approximately 210 million Euros upon completion of the transaction. The transaction, which is subject to customary closing conditions, is expected to be completed in July 2018.
“I am very pleased that Philips will become the home for our innovation, our business and our people,” said Professor Shlomo Ben-Haim, founder and chairman of EPD Solutions. “Philips’ expertise and leadership in interventional imaging and navigation is an excellent strategic fit with EPD. I am convinced that as part of Philips, we will be able to grow EPD and help many electrophysiologists and patients worldwide, as we aim to reduce procedure costs, simplify navigation and treatment, and ultimately improve procedure efficacy.”
The combination of Philips’ interventional imaging systems, such as Azurion, and EPD’s cardiac imaging and navigation system provides the maps and data used by EPs to guide various catheters to locate and treat cardiac arrhythmias.
EPD’s system, which obtained European CE marking in February, 2018, is based on proprietary software algorithms and single-use electromagnetic sensors, used in conjunction with standard electrophysiology catheters. The system has been installed at several leading hospitals across Europe. A U.S. Food and Drug Administration 510(k) premarket review for the system is currently under review. The system is not available for sale in the U.S.
Upon completion of the transaction, EPD and its employees will become part of Philips’ Image-Guided Therapy business. Professor Shlomo Ben-Haim, who has a proven track record in building successful medical device businesses, will continue to support Philips to build a new business based on this acquisition.
For more information: www.usa.philips.com


 February 06, 2026
February 06, 2026 









