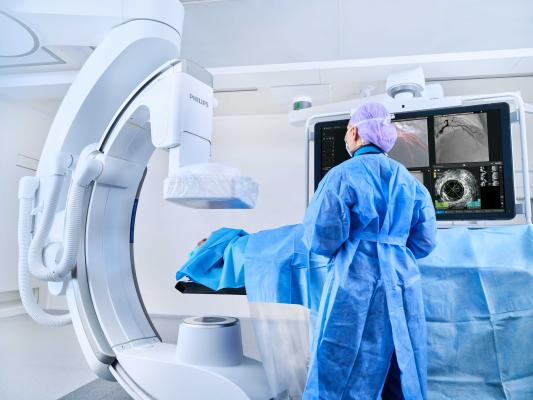
May 20, 2025 — Royal Philips has launched the RADIQAL (Radiation Dose and Image Quality Trial) trial. This multicenter, randomized study, sponsored by Philips will enroll 824 coronary artery disease patients across 6 hospitals in Spain, Czech Republic and the US. The first patient in the study was enrolled at Aarhus University Hospital, Denmark.
“The ability to reduce radiation exposure without compromising procedural performance is a key priority in interventional cardiology,” said Dr. Javier Escaned, professor of Cardiology at Hospital Clínico San Carlos and principal investigator. “It is also important to achieve high-quality angiograms when using diluted contrast media as part of ultra-low contrast procedures. RADIQAL is designed to generate robust, real-world evidence on whether Philips’ new ultra-low X-ray dose technology can reduce radiation exposure for patients and staff without affecting the quality of coronary procedures.
Coronary artery disease (CAD) is the most frequent type of heart disease affecting millions of people worldwide. It is caused by chronic inflammation of the coronary arteries, which may lead to a gradual obstruction or sudden occlusion of blood flow to the heart muscle. Percutaneous coronary intervention (PCI) is a widely used image-guided, minimally invasive procedure to open blocked coronary arteries and treat CAD. Philips Azurion is an image-guided therapy system which is used for live X-ray imaging during such procedures.
The RADIQAL trial evaluates radiation exposure, image quality and procedural performance between Philips’ new ultra-low dose technology and existing ClarityIQ technology, both integrated into the Azurion image-guided therapy system. The new technology features an ultra-low dose protocol for coronary procedures, reducing X-ray exposure by 50% compared to even the lowest setting currently available on our Azurion systems with ClarityIQ. This technology has obtained CE marking and as such is cleared under the EU MDR regulatory framework*.
“Reducing radiation exposure while maintaining or improving image-quality is one of the most important innovation goals in interventional cardiology,” said Dr. Darshan Doshi, Head of Medical & Clinical at Philips Image-Guided Therapy Devices and Interventional Cardiologist at the Massachusetts General Hospital in Boston, USA. “Interventional cardiologists rely on low-dose, high-quality imaging for confident decision-making throughout multiple procedures each day. Also for patients, especially those with high BMI or with complex conditions requiring repeat interventions, minimizing radiation exposure is increasingly critical.”
* Not cleared as a medical device in FDA-regulated countries. Enrollment in the US has not started.


 January 28, 2026
January 28, 2026 









