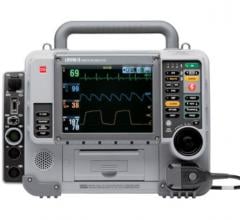
September 4, 2007 – Philips recently demonstrated its HeartStart MRx monitor/defibrillator that enables paramedics to transmit patient data from the ambulance to the hospital’s emergency department, at the European Society of Cardiology’s (ESC) annual congress in Vienna, Austria.
Upon reception of the data at the hospital, clinicians can use the ECG data to begin assessing what treatment the incoming patient will need. By allowing a hospital to begin organizing its resources before the patient arrives, the MRx can reportedly help reduce the time to treatment.
In addition to transmitting ECG data to the hospital prior to the patients’ arrival, the HeartStart MRx integrates with the hospital’s ECG management system TraceMasterVue, enabling critical patient information to be seen where it’s needed.
For more information: www.philips.com


 January 13, 2026
January 13, 2026