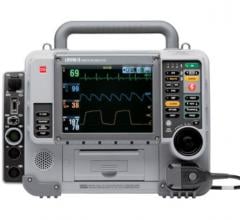
October 6, 2008 - Physio-Control Inc. last week announced FDA clearance to market the LIFEPAK 20e defibrillator/monitor in the U.S.
The 20e is an enhancement of the LIFEPAK 20 defibrillator/monitor, which has become the standard of care in many hospitals worldwide since its introduction in 2002. It offers all the capabilities of the LIFEPAK 20 device, along with a more powerful lithium-ion battery that doubles ECG monitoring time and the run time of other parameters such as noninvasive pacing and pulse oximetry, a noninvasive way to monitor the oxygenation of a patient’s hemoglobin. Additionally, a new on-screen “fuel gauge” displays the real-time status of available battery capacity so clinicians can monitor remaining use time.
The 20e is an enhancement of the LIFEPAK 20 defibrillator/monitor, offering a more powerful Lithium-ion battery that doubles ECG monitoring time and the run time of other parameters such as noninvasive pacing and pulse oximetry.
Physio-Control is currently under a consent decree with the FDA, which restricts the amount of products that can be delivered to customers. However, the company is permitted to manufacture and ship a limited selection of product under certain conditions to meet the critical needs of emergency response customers.
For more information: www.physio-control.com


 January 13, 2026
January 13, 2026