May 13, 2021 — PolyVascular, leading the effort to improve children's lives with a novel polymeric transcatheter valve (PTV) for children, announced that it was awarded a $2 million Small Business Innovation Research (SBIR) Phase II grant earlier this year to bring its polymeric stent-mounted valve (SMV) to clinical trials within the next two years in an effort to address pediatric congenital heart disease (CHD), which affects over 1 million patients around the world.
As part of the next stage of growth for the company, PolyVascular named Will Clifton, M.D., as Chief Operating Officer (COO) to oversee the completion of the grant as Principal Investigator and to manage the operations and R&D for the company.
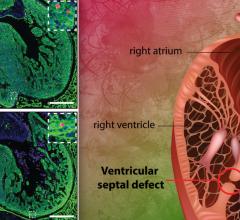
PolyVascular’s SMV is composed of polymer leaflets mounted within a metal stent that can be delivered via a minimally invasive transcatheter procedure, avoiding the risks of surgery, with potential for improved durability and function. Congenital heart disease remains the most common category of birth defect and a leading cause of childhood death in the developed world. Dysfunctional pulmonary valves (PV) are a common abnormality, and frequently require surgical intervention and replacement. Valve replacement through open heart surgery carries substantial risk and discomfort for pediatric patients, and represents a major financial and emotional burden for families. The most commonly used valves for pulmonary valve replacement in young children are biologically-derived (e.g. human cadaveric valves or biological tissue from animals), but are in short supply and have a tendency to not last very long, leading to repeat surgeries.
“Our aim at PolyVascular is to transform the care of children with congenital heart disease by developing an entirely new generation of valves made of medical grade polymer devoid of any biological tissue,” said Henri Justino, M.D., Chief Medical Officer, PolyVascular. “The valves in use so far for children have been made of biological tissue. Unfortunately, our immune systems target and destroy this biological tissue, sometimes rapidly, rendering the valve ineffective. PolyVascular’s approach will allow us to deliver these valves in a minimally invasive fashion, and extend the time between repeat surgeries. We are excited about our growth trajectory over the next few years, but we are more excited to make a difference in the lives of children with CHD and their families.”
Children age five and older will be eligible for the trans-catheter technology which will place the PTV device in the heart. The polymer valves last longer and can stay in the heart for longer versus biological valves which need to be replaced every few years as the child grows. When the polymer valves need to be replaced with larger sizes, the procedures will be done via catheter-based surgeries which save the patient from more invasive open-heart surgeries.
“I am very honored and excited to lead PolyVascular’s efforts to bring this brand new polymeric transcatheter valve to in-human trials within the next two years and then ultimately to the first pediatric patients,” said Will Clifton, M.D., COO of PolyVascular. “Our polymer-based valve will give pediatric patients around the world a greater quality of life as well as greater peace of mind for their families because of the durability of the valves.”
Clifton served as Sr. Director of Medical Affairs, at Procyrion, a clinical-stage medical device company in Houston. Will is also the President and co-founder of Enventure, a Houston medical innovation incubator and education hub. Will has also served as Director of Medical Innovation and lecturer at Rice University in the Department of Bioengineering and teaches the Biodesign Fellowship for TMC Innovation.
For more information: www.polyvascular.com


 March 31, 2025
March 31, 2025