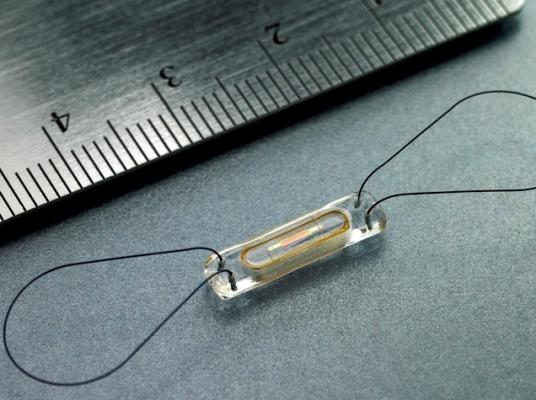
June 24, 2015 - Scott & White Memorial – Temple for the first time implanted a new miniaturized, wireless monitoring sensor to help manage heart failure (HF). Scott & White Memorial is one of six hospitals in Texas and the first hospital in the Baylor Scott & White Health system to offer the device.
"We are always looking for new and innovative ways to treat our patient's disease process and improve outcomes for heart failure patients," said Robert Scott III, M.D., director for advanced heart failure at Scott & White Memorial. "This device will give us the ability to anticipate problems with our patients before they occur, decreasing their chance of being re-admitted to the hospital, and improving their quality of life."
Memorial implemented the CardioMEMS HF System, a U.S. Food and Drug Administration (FDA)-approved heart failure monitoring device proven to significantly reduce hospital admissions.
The device is a sensor that is implanted in the pulmonary artery (PA) during a minimally invasive procedure. Once implanted, the device can measure and transmit PA pressure from the patient back to their healthcare team. Elevation in PA pressure appears even before changes in weight and blood pressure in the patient, which are traditionally used as indirect measures of worsening heart failure. The new system allows patients to transmit daily sensor readings from their homes to their healthcare providers allowing for personalized and proactive management to reduce the likelihood of hospitalization and onset of debilitating symptoms.
"Heart failure can rob patients' quality of life and frequently results in repeated hospitalizations," said John Erwin III, M.D., cardiologist at Scott & White Memorial. "We think that we can provide significantly improved quality of life by partnering with the patient in acting preventatively as opposed to responding when an adverse event occurs."
For more information: www.sw.org


 February 03, 2026
February 03, 2026 









