May 18, 2012 -- A study conducted at Scripps Health has found that a novel new heart monitoring device helped emergency room patients avoid unnecessary follow-up care. Scripps Health electrophysiologist Steven Higgins, M.D., presented findings of the study titled, "Prevalence of Arrhythmias in Emergency Department Patients Discharged Using a Novel Ambulatory Cardiac Monitor,"at the Heart Rhythm Society's 33rd Annual Scientific Sessions in Boston.
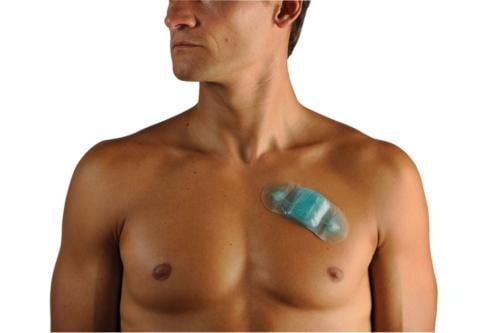
The study focused on the use of Zio Patch, a single-use ambulatory cardiac monitor that looks similar to a 2- by 5-inch adhesive bandage and sticks to a patient's chest. The device can continuously monitor their heart rhythm for up to 14 days.
"The availability of this new heart monitor is exciting as it improves patient care. The patch is applied and when recording is done, the patient simply drops it in the envelope and returns it through the mail – it's like the Netflix of heart care," said Higgins, chairman of the department of cardiology at Scripps Memorial Hospital La Jolla and a lead investigator. "Because they are infrequent, heart rhythm problems are often difficult to diagnose, even though they can be quite serious. The Zio Patch is a new digital advance that will allow us to better diagnose challenging cases so we can provide our patients the best care."
Scripps Memorial Hospital La Jolla was the only hospital in Southern California to participate in the study. Other study locations included Stanford Hospital and Scott & White Memorial Hospital in Temple, Texas.
The study followed 285 patients who had presented to emergency departments across the country with symptoms possibly related to arrhythmias, such as fainting, palpitations, dizziness and others. Patients received the unencumbering, wire-free Zio Patch prior to being discharged from the emergency room and were instructed to wear the patch until it no longer adhered to their skin – up to 14 days duration. Devices were mailed back to iRhythm Technologies, Inc., the Zio Patch's developer and service provider, using a pre-paid postage envelope, for analysis and reporting of results to the patient's physician.
The researchers found that 59 percent of the symptomatic patients who presented to the emergency rooms did not have arrhythmia and may not require any further work-up. "Thus, the new device has the potential to save the health care system millions of dollars," said Higgins. "We were also surprised to learn that there was 100 percent compliance by the patient with the process, which is an amazing finding for an emergency department study."
Monitoring Leads to Possible Lifesaving Procedure
One patient who benefited from the Zio Patch is La Jolla resident, Kenneth Curzon, who fainted while at work in March. Curzon continuously wore the Zio Patch for two weeks and then mailed it back to iRhythm, where the information was downloaded and formatted into a report for Higgins to review.
The recording showed Curzon was experiencing prolonged pauses in his heart rhythm of over three seconds as well as other episodes of rapid heart beats. On April 6, he received an implantable cardiac defibrillator to correct the problem and was back to his management job within five days.
"The Zio Patch allowed me to diagnose and determine the most appropriate therapy for Ken," said Higgins.
"I like to think of the whole experience as an adventure," Curzon said. "Most of the time I didn't even realize I was wearing a heart monitor, and when I peeled it off, I just put it in an envelope and sent it off in the mail. It was a very simple process."
Zio Patch vs. Holter Monitor
In addition, Eric Topol, M.D., is leading a new related study at Scripps Green Hospital examining whether the Zio Patch does a better job of detecting heart arrhythmias than the Holter monitor, which has been the gold standard for rhythm monitoring since the early 1960s.
The portable Holter monitor collects its data through a series of wired electrodes that adhere to the chest. Because the device can be difficult to wear and can get in the way of normal activities such as showering, exercising and sleeping, continuous use of the monitor is typically limited to one or two days. In contrast, the Zio Patch is a small, unobtrusive device that is indicated for up to 14 days of wear, and allows the patient to exercise and shower on their normal schedule, without the hassle of a bulky monitor and multiple wires.
"This is a great opportunity to compare these two side by side for use in diagnosing important heart rhythm disturbances," said Topol, a cardiologist who directs the Scripps Translational Science Institute and serves as chief academic officer of Scripps Health. "We are trying to determine if the Zio Patch will have an increased diagnostic yield."
The study is currently enrolling about 150 Scripps Green and Scripps Clinic adult patients who have been seen by their doctors for arrhythmia. Each of the participants will wear a Holter monitor and a Patch for up to 48 hours and then continue wearing the Zio Patch for up to 14 days.
Topol and his research associates will compare the data gathered from each device and report their findings later this year.
Arrhythmias affect millions of Americans each year and, if left untreated, may lead to serious consequences including stroke or sudden cardiac death.
More information about the study is available at www.clinicaltrials.gov


 January 15, 2026
January 15, 2026 









