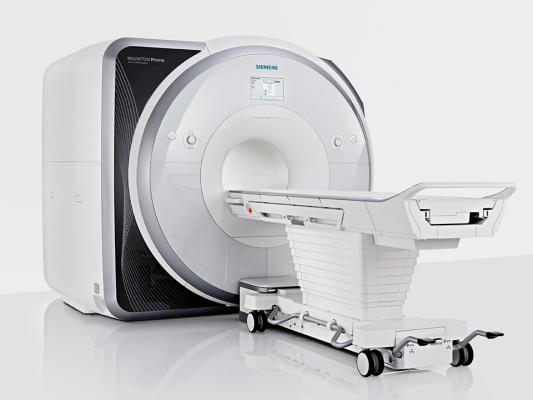
May 12, 2014 — The Center for Magnetic Resonance Research (CMRR) at the University of Minnesota, Minneapolis, is the first U.S. facility to install Siemens Healthcare’s Magnetom Prisma, a 3T magnetic resonance imaging (MRI) scanner capable of exceptionally high spatial and temporal resolution to achieve image quality, particularly in highly demanding situations.
“The Center for Magnetic Resonance Research is excited to add the Magnetom Prisma 3T scanner to support our research efforts to obtain better information about anatomical connections within the brain.” said Kamil Ugurbil, Ph.D., director of CMMR and a professor in the departments of radiology, the neurosciences and medicine at the University of Minnesota. “The large gradient strength in the Prisma provides the potential for us to encode the diffusion of water in the brain much more quickly than conventional MRI scanners, yielding signal-to-noise gains that increase image resolution and enhance our ability to detect smaller effects in the brain.”
The Magnetom Prisma possesses XR Gradients, which combine 80 milliTesla per meter (m/T/m) with a 200T-per-meter-per-second (T/m/s) slew rate within one sequence for a configuration not found on any other commercial whole-body MR system. Complementing it is the system’s highly robust design, which enables precision to be maintained through long, demanding applications such as functional MRI (fMRI). The power and endurance of the Magnetom Prisma’s XR Gradients fuel the system’s first-to-market Diffusion Spectrum Imaging (DSI) acquisition, which enables the user to potentially resolve fine anatomical details of the brain such as crossing white-matter fibers.
Further enhancing the Magnetom Prisma’s image quality is the scanner’s benchmark homogeneity and advanced shimming solutions that allow for finer, more effective compensation of patient-induced field disturbances. Combined, these features offer the user an outstanding basis for cardiac and body imaging, as well as applications such as spectroscopy.
The Magnetom Prisma possesses Siemens’ Dot (Day optimizing throughput) engines, which enable the high level of consistency that is crucial for both research applications and demanding clinical work. The Dot engine brings automation and reproducibility to investigations across subjects, time points and institutions to raise the integrity of longitudinal studies and multisite research projects.
For more information: www.usa.siemens.com/healthcare


 January 21, 2026
January 21, 2026 








