October 28, 2013 — Simbionix, a provider of training solutions, announced the initiation of the REHEARSAAAL study which evaluates the clinical benefits of preoperative patient specific simulation using the PROcedure Rehearsal Studio for the treatment of Abdominal Aortic Aneurysms (AAAs) by Endovascular Aortic Repair (EVAR). A multidisciplinary group of physicians from vascular surgery, interventional radiology and cardiology departments in hospitals across the United States and Europe will conduct the study.
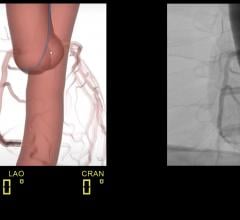
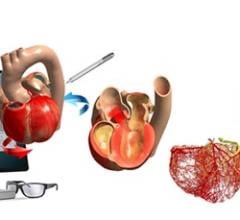
PROcedure Rehearsal Studio allows physicians to create a 3-D anatomical model based on a patient's individualized computed tomography CT scan to simulate, analyze and evaluate preoperative surgical options. The availability of patient specific technology allows the interventional team to engage in simulated practice procedures utilizing a patient's own anatomy prior to performing an actual procedure, potentially leading to more precise treatment decisions, a reduction of errors, improved outcomes and enhanced operator confidence. Prior case simulation may allow patients to have a shortened procedure with less time under sedation, a reduction in radiation exposure and a decrease in procedural complications.
This two-arm study will randomize 150 patients in a one-to-one ratio, stratified by operator's experience and procedure complexity. It compares real EVAR procedure parameters with an emphasis on radiation exposure time and safety parameters. Physicians treating patients enrolled for the preoperative patient specific simulation group will go through the simulated experience — a virtual practice session — prior to performing the real case. Outcomes in these patients will be compared to procedures performed without prior simulated practice as is routinely done.
“Simulation has proven experience in the aviation and other industries in improving performance and reducing errors,” said John Rundback, M.D., medical director, Interventional Institute, Holy Name Medical Center, Teaneck, N.J. and the study's principal investigator. “The Simbionix PROcedure Rehearsal Studio provides a platform to practice and optimize procedures using personalized patient data in advance of performing live cases, assuring an unparalleled opportunity to streamline all aspects of the actual procedure, improve patient safety and reduce the use of additional costly devices that are often needed when procedures are unable to be rehearsed."
Guests of the Transcatheter Cardiovascular Therapeutics annual conference (TCT 2013) can see the PROcedure Rehearsal Studio at booth 2408. Guests of the Vascular and Endovascular Issues, Techniques, and Horizons (VEITHsymposium) can see it at booth 110.
For more information: www.simbionix.com


 March 11, 2022
March 11, 2022