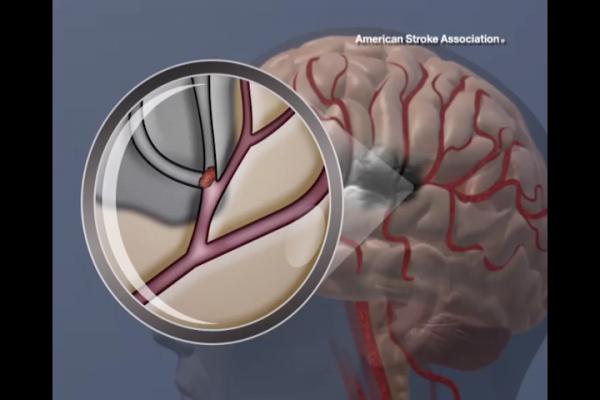
March 24, 2015 — A study in the March 24/31 issue of JAMA found that using balloon-expandable stents to treat symptomatic intracranial arterial stenosis resulted in an increased risk of stroke or transient ischemic attack (TIA). The study results support the use of medical therapy (clopidogrel and aspirin) as an alternative to balloon-expandable stents.
Intracranial arterial stenosis (narrowing of an artery inside the brain) is a common cause of stroke worldwide. The recurrent stroke risk with severe symptomatic intracranial stenosis may be as high as 23 percent at 1 year, despite medical therapy, according to background information in the article.
Osama O. Zaidat, M.D., M.S., of the Medical College of Wisconsin/Froedtert Hospital, Milwaukee, and colleagues randomly assigned 112 patients with symptomatic intracranial stenosis (narrowing of 70 percent or greater) to receive a balloon-expandable stent plus medical therapy (stent group; n = 59) or medical therapy alone (medical group; n = 53). Medical therapy consisted of clopidogrel (75 mg daily) for the first 3 months after enrollment and aspirin (81-325 mg daily) for the study duration.
This international trial (VISSIT) enrolled patients from 27 sites (January 2009-June 2012) with last follow-up in May 2013. Enrollment was halted by the sponsor after negative results from another trial prompted an early analysis of outcomes, which suggested futility after 112 patients of a planned sample size of 250 were enrolled.
The 30-day safety end point of any stroke within 30 days or hard TIA within 2 to 30 days was 9.4 percent (5/53) in the medical group and 24.1 percent (14/58) in the stent group; TIA was defined as a transient episode of neurological dysfunction caused by focal brain or retinal ischemia lasting at least 10 minutes but resolving within 24 hours. Ischemic stroke was observed in three patients (5.7 percent) in the medical group and in 10 patients (17.2 percent) in the stent group. Intracranial hemorrhage occurred in five patients (8.6 percent) in the stent group and in 0 in the medical group. The 1-year outcome of stroke or hard TIA occurred in more patients in the stent group (36.2 percent) vs the medical group (15.1 percent).
Thirty-day all-cause death was 3 of 58 patients (5.2 percent) in the stent group and 0 in the medical group. A measure of disability worsened in more patients in the stent group than in the medical group.
“These findings do not support the use of a balloon-expandable stent for patients with intracranial arterial stenosis,” the authors conclude.
For more information: www.media.jamanetwork.com


 February 02, 2026
February 02, 2026