
The Maquet CardioHelp System
Mr. KH is a 58-year-old male with a past medical history of hypertension and tobacco abuse who presented to the emergency department (ED) with an episode of syncope. The patient stated that he was at home walking to the kitchen when he felt light-headed and then woke up on the floor. The event was witnessed by his wife, and he was not noted to have any seizure-like activity. On arrival in the ED, the patient began to complain of chest discomfort and dyspnea. A stat electrocardiogram (ECG) revealed sinus rhythm and 2 mm ST-segment depression in leads V4-V6. The patient lost consciousness while in the ED and was emergently intubated. His blood pressure was 65/45, and he was started on intravenous phenylephrine, dopamine and norepinephrine to maintain a blood pressure of 70/40. The patient was taken emergently to the cardiac catheterization laboratory (CCL) where an intra-aortic balloon pump (IABP), was emergently placed. Coronary angiography revealed mild atherosclerotic disease and the aortic valve could not be crossed.
A post-procedure transthoracic echocardiogram (TTE) demonstrated a left ventricular ejection fraction of 25 percent with global hypokinesis and significant aortic stenosis with a gradient of 30.4 mmHtg, a velocity of 3.71 m/sec across the aortic valve, and a calculated aortic valve area of 0.41 cm2.
Given his profound hypotension on multiple intravenous pressors and the IABP, Mr. KH was emergently transferred to the University of Chicago Medical Center. On arrival, the patient was in profound cardiogenic shock with a heart rate of 122 beats per minute and a blood pressure of 72/42 while on intravenous dopamine, dobutamine, phenylephrine, norepinephrine and vasopressin. He was intubated and had an arterial saturation of 85 percent. On exam, he was cool to touch with no response to noxious stimuli. He had few audible breath sounds and his cardiac exam was significant for tachycardia and a harsh systolic ejection murmur. His laboratory values were significant for a white blood cell count of 40.2 K/uL (normal 3.5-11), a lactic acid of 9.5 mEq/L (normal 0.7-2.1) and an AST/ALT of 890/450 U/L (nl 8-37). His chest radiograph revealed cardiomegaly, bilateral pleural effusions and pulmonary congestion. His ECG revealed atrial fibrillation with a rapid ventricular response, ST-segment elevation in aVR and 3 mm ST segment depression in I, aVL, V4-V6.
The patient was taken emergently to the cath lab. In the cath lab, repeat coronary angiography was performed to evaluate for any interval change in the coronaries and there was none. A catheter was placed in the left ventricle and the intracavitary pressure was 185/15 mmHg with simultaneous aortic pressure recording of 69/34 with a mean gradient of 82 mmHg. His critical aortic stenosis was thought to be the etiology for his syncope and profound cardiovascular collapse. Cardiothoracic surgeons deemed that he was too high risk to undergo open surgical aortic valve replacement so a decision was made to proceed with percutaneous balloon valvuloplasty with hemodynamic support. With both respiratory and circulatory failure, we decided to support the patient with the Maquet CardioHelp system — see Figure 1.
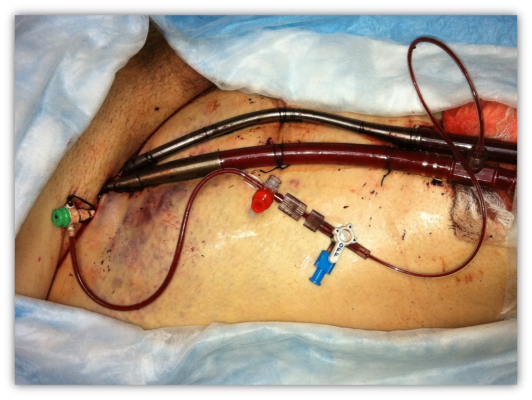
A 6 French sheath was placed in the right common femoral artery. Using ultrasound guidance, a 6 French sheath was placed in an antegrade fashion into the right common femoral artery for distal limb perfusion. A 10 French sheath was placed in the right common femoral vein. A 25 French, 60 mm venous cannula was advanced over a 0.035 x 260-inch stiff wire into the superior vena cava with fenestrations straddling the right atrium and the inferior vena cava. A 17 French 18 mm arterial cannula was placed over the stiff wire into the right common iliac with the side port connected to the antegrade sheath (Figure 2). Flows were initiated at 4.5 L/min.
Through a 12 French sheath, a 22 x 40 mm valvuloplasty balloon was used to dilate the aortic valve. After two dilations, the mean gradient across the aortic valve decreased to 17 mmHg. The patient’s arterial pressure rose to 98/80 mmHg with a mean of 85 mmHg and the vasopression, phenylephrine and norepinephrine were weaned off prior to the patient leaving the cath lab.
Immediate post-procedure TTE revealed a left ventricular ejection fraction of 45 percent, a mean gradient across the AV of 17 mmHg, a velocity of 1.2 m/sec and an aortic valve area of 1.3 cm2.
After two days on the CardioHelp, the patient was weaned off all intravenous pressor agents, his chest X-ray revealed improved pulmonary congestion and his FIO2 decreased from 100 percent on admission to 40 percent. The patient was taken to the operating room where he received a 25 mm Trifecta valve and underwent decannulation of the femoral cannulae.
He was successfully extubated and discharged to an inpatient rehabilitation facility on post-operative day seven.
This case demonstrates the role of the Maquet CardioHelp system as an optimal mechanical cardiopulmonary support device during high-risk balloon valvuloplasty. The system facilitated cardiac recovery and served as a bridge to definitive surgical aortic valve replacement.
Caption for Figure 2: A 6 French sheath was placed in the right femoral artery. A 6 French sheath was placed in an antegrade fashion into the right common femoral artery for distal limb perfusion. A 10 French sheath was placed in the right common femoral vein. A 25 French, 60 mm venous cannula was placed in the superior vena cava with fenestrations straddling the right atrium and the inferior vena cava. A 17 French 18 mm arterial cannula was placed in the right common iliac with the side port conn.


 June 19, 2024
June 19, 2024 









