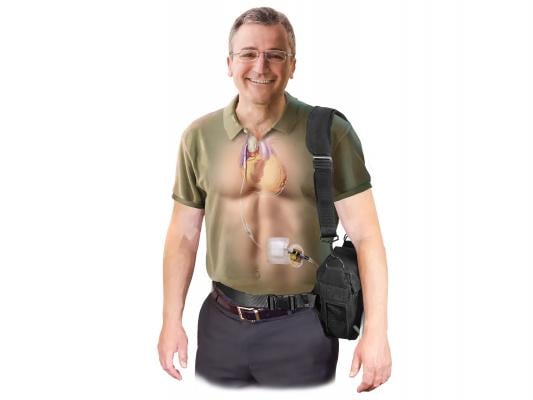
August 3, 2015 — Sunshine Heart announced plans to commence a first-in-human study using its novel C-Pulse transcutaneous energy transmission (TET) system and a new, smaller implantable pump in quarter three 2016.
The study will initially enroll patients at hospitals outside the United States, and will target patients suffering from advanced Class III/IV heart failure, similar to the patient population of Sunshine Heart's current ongoing COUNTER HF Study. Feasibility of implant, safety and short-term durability will be the primary objectives. Performance and efficacy data will be collected to evaluate patient quality of life, freedom from heart failure symptoms and other standard heart failure measures including the ability to bridge to other therapies.
William Cohn, M.D., director of the Center for Technology and Innovation, Texas Heart Institute, who has been involved since the inception of the program, will lead the study. Cohn commented, "The development of a completely implantable circulatory assist device that obviates the need for a driveline will be an important milestone in the evolution of heart failure therapies. The system will also be non-blood contacting and non-obligatory and may prove to be a real game changer for patients with intermediate stage heart failure."
The development of a fully implantable system will eliminate the need to manage a driveline and will address the risk of exit site infections. Together with smaller and lighter wearable components, the system is designed to provide an improved quality of life for patients. The company believes this system will lead to increased market access to target all Class III HF patients, similar to cardiac rhythm management (CRM) and euromodulation devices. In addition, the unique ability to deliver chronic counterpulsation therapy and augment coronary blood flow, may afford a new market opportunity for the treatment of angina and ischemic heart disease where morbidity and mortality remain high in heart failure populations with reduced and preserved ejection fraction.
The benefits of C-pulse and counterpulsation on the pulmonary circulation and the high prevalence of pulmonary hypertension in the latter population may also provide for a therapeutic opportunity with a fully implantable system in this large population with an unmet need.
For more information: www.sunshineheart.com


 February 03, 2026
February 03, 2026 









