August 19, 2010 – The 12-month follow-up results from the TITAN trial of the Carillon Mitral Contour System will be presented Aug. 29 at the European Society of Cardiology 2010 Congress (ESC) in Stockholm. Professor Uta Hoppe of the University of Cologne, Germany, will present the 12-month trial data and review the safety and efficacy of the system, a novel therapy for treating heart failure patients suffering from functional mitral regurgitation (FMR).
Most of the estimated 5 million people in the United States and more than 20 million people worldwide suffering from heart failure also suffer from FMR, along with dilated cardiomyopathy. Unfortunately, most of these patients have few options and are inadequately treated using medical management. While surgical options exist and can be effective in reducing FMR, they are infrequently used due to the burden of the surgery itself, which can be associated with high operative morbidity and mortality rates.
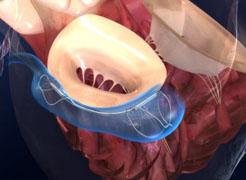
The Carillon Mitral Contour System is a nonsurgical, minimally invasive device designed to repair the mitral valve in these patients and reduce FMR. It combines a proprietary implantable device and a percutaneous delivery system.
The 12-month follow-up trial results are highly anticipated because the previously released six-month data presented at TCT 2009 was very promising, said professor Dr. Michael Haude of the Stadtische Kliniken Neuss, Lukaskrankenhaus, Germany, one of the study investigators.
"In my experience this device presents key advantages, such as an easy-to-learn technique and immediate assessment of efficacy coupled with periprocedural recapturability, if necessary," Haude said. "We found that the majority of patients experienced immediate clinical improvement, but importantly, the clinical and hemodynamic improvement was sustained through the one year of follow-up. The clinical data also suggests that the Carollon device does not preclude later use of complementary therapies, such as biventricular pacing."
The trial protocol evaluated the clinical impact of coronary sinus-based percutaneous mitral annuloplasty in symptomatic patients with both ischemic and nonischemic dilated cardiomyopathy, at least moderate FMR, and stable medical therapy. Anatomical exclusions were limited to those patients who had significant mitral leaflet pathology or severe mitral annular calcification. Safety was evaluated as the one-month composite major adverse event (MAE) rate. Hemodynamic efficacy was measured by an echocardiographic core lab, which assessed left ventricular dimensions, function and quantitative measures of FMR. Functional efficacy was assessed by exercise testing as well as quality-of-life metrics.
The maker of the device, Cardiac Dimensions, hopes to move forward with European commercialization and push forward with the U.S. investigational plan.
For more information: www.cardiacdimensions.com


 December 24, 2025
December 24, 2025 









