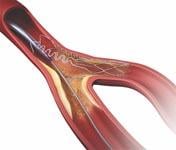
September 15, 2009 – Tryton Medical Inc. said today its Tryton Side Branch Stent has been used in 250 procedures to treat atherosclerotic lesions at bifurcations in patients with heart disease.
“Bifurcation lesions have presented a challenge for cardiologists since the earliest days of angioplasty,” said professor David Foley of Beaumont Hospital in Dublin, Ireland (formerly the cardiac catheterization lab director at Thoraxcentre, Rotterdam). “Current approaches to treating these cases entail adaptation of available stents, leading to complexity and increased risk of periprocedural complications and late restenosis. The Tryton Side Branch Stent offers a straightforward approach to first securing the side branch, while enabling trouble-free stenting of the main vessel using either a drug eluting or bare metal stent. Over the past two months, I have subjected the Tryton Stent to robust challenges in complex bifurcations and am happy to state that it has become an important tool for treating my patients with bifurcation lesions.”
Tryton Medical will exhibit the Tryton Side Branch Stent System in booth 630 at this year’s Transcatheter Cardiovascular Therapeutics (TCT) Conference Sept. 21-25, in San Francisco.
Tryton’s highly deliverable cobalt chromium stent is deployed in the side branch artery using a standard single-wire balloon-expandable stent delivery system. A conventional drug-eluting stent is then placed in the main vessel.
The Tryton Side Branch Stent System demonstrated excellent six-month clinical results in a first-in-man study of the system in 30 patients, with no restenosis occurring in the side branch artery. The stent received CE mark approval in Europe, but is not approved in the United States.
For more information: www.trytonmedical.com


 November 24, 2025
November 24, 2025 









