May 24, 2012 — Research led by Christiana Care Health System's Andrew Doorey, M.D., has found that a small number of patients mistakenly undergo bypass surgery after diagnostic angiography.
The findings — presented May 10 in Las Vegas during the Society for Cardiovascular Angiography and Interventions' annual meeting — could determine a new way of clinical practice that could save patients from unnecessary operations and reduce healthcare costs.
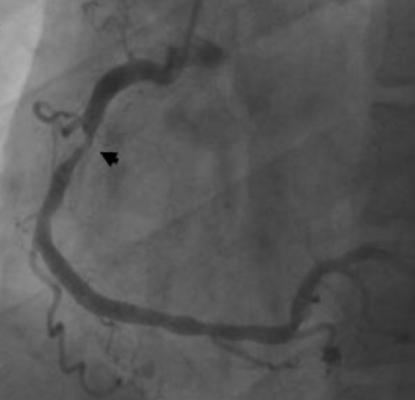
The findings showed that some patients are erroneously diagnosed with having stenosis when they in fact have experienced a vasospasm.
Stenosis is the hardening and narrowing of the arteries. It is caused by a slow and progressive buildup of plaque under the lining of the arterial wall. Vasospasms, on the other hand, are spasms in the blood vessels' walls that are induced by a coronary catheter during a diagnostic angiography, a procedure that uses a special dye and X-rays to see how blood flows through the heart.
There is a difficulty in distinguishing a temporary blockage from a fixed blockage because both look identical, said Doorey, the study's principal investigator and a cardiologist with Christiana Care Cardiology Consultants. But the problem of the vasospasm in the critical left main artery had never been reported before and is now being brought to light.
Researchers examined 2,313 patients over a 10-year period. Among them, 385 patients had a subsequent catheterization at Christiana Hospital.
Sixteen of those 385 patients showed no signs of stenosis on a repeat study. Six of those 16 patients, however, underwent a coronary artery bypass graft surgery, the most commonly-performed open-heart operation to bypass blockages and obstructions. Since stenosis restricts bloodflow to the heart, a patient diagnosed with the condition is often immediately referred for surgery.
Instead of directing a patient with signs of stenosis immediately to surgery, doctors should take measures to ensure the patient be given intracoronary nitroglycerin, which can successfully treat the vasospasm. If the patient does not respond to the nitroglycerin, there is a stronger indication that the patient indeed has stenosis.
For more information: www.surgprodmag.com


 January 15, 2026
January 15, 2026 









