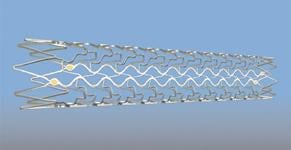
September 18, 2009 – Following the recent CE mark certification of the vProtect Luminal Shield, the novel self-expanding coronary stent system will be the focus of scientific presentations and roundtable discussions on lesion-specific therapy for coronary artery disease at TCT 2009, Sept. 21-25, in San Francisco. The vPredict Optical Catheter System, a diagnostic device to detect plaque composition, also from Prescient Medical, will also be featured in several presentations.
All sessions will be held at the Moscone Center.
• 8 a.m. to 6:45 p.m., Monday, Sept. 21, room 131 – Both devices will be discussed in the day-long scientific symposium "Vulnerable Plaque: Emerging Direction for Diagnosis and Treatment." Professor Patrick W. Serruys of Erasmus Medical Center in Rotterdam will discuss clinical experience with the vProtect Luminal Shield in stabilizing vulnerable plaque at 5:05 p.m. "Recent developments in the field of vulnerable plaque are very exciting," said Dr. Serruys. "We are finally making progress in characterizing, understanding, and potentially treating these lesions." Other key presentations include discussions of preclinical and clinical studies by Juan Granada, M.D., of the Cardiovascular Research Foundation, and Giuseppe Sangiorgi, M.D., director of interventional cardiology at the University of Rome at 9 a.m., 3:30 p.m., and 4:45 p.m.
• 4-4:20 p.m. Wednesday, Sept. 23, room 134 – Dr. Granada, will present key findings relating to the low-injury self-expanding design of the vProtect Luminal Shield during "Coronary Stent Design and Device Development."
• 4:45-4:55 p.m. Thursday, Sept. 24, room 133 – Dr. Granada will present additional data on the vProtect Luminal Shield during "The Re-Emergence of Nitinol Self-Expanding Coronary Stents," a special session dedicated to exploring the re-emergence of self-expanding coronary stents led by the vProtect Luminal Shield. "It has become clear that stent designs and materials should be tailored to the pathophysiology of the lesion to be treated,” Dr. Granada said. “The Shield is designed specifically to minimize arterial injury and its consequences, making it ideal for softer plaques."
For more information: www.prescientmedical.com


 November 24, 2025
November 24, 2025 









