December 11, 2009 – A new nonlinear algorithm has been selected for a month-long April 2010 noninvasive cardiac screening of teenagers in danger of sudden cardiac death in south east London. Vicor Technologies Inc. said its PD2i nonlinear algorithm will be used along with ECG and echocardiogram to identify those at risk.
Dr. Sanjay Sharma, consultant cardiologist of cardiac risk in the young, is spearheading the screening initiative that will offer free cardiac screenings to 14 year olds living in South East London. Studies show 14 is the earliest post-puberty age for which proactive screening is considered viable. The ultimate goal of the initiative is to develop a prototype for a national screening program for young sudden cardiac death. About 600 young people are struck down by sudden cardiac death annually in the U.K.
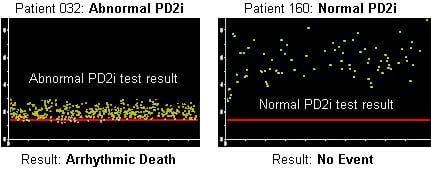
The company said its algorithm gives consistently accurate results in risk stratifying adults at for sudden cardiac death.
Dr. Sharma was recently-appointed professor of the newly-created Inherited Cardiovascular Disease and Sports Cardiology Centre at St. George’s Healthcare NHS Trust. He will begin his tenure in February 2010. The Centre is the world’s first specialist, multidisciplinary facility dedicated to providing services to populations affected by or at risk for (including families and athletes) young (14-35) sudden cardiac death. St. George’s is one of England’s largest teaching hospitals and the first hospital in the United Kingdom to develop a specialist clinic for young sudden cardiac death.
Vicor is a development-stage biotechnology company creating noninvasive diagnostics employing its patented, proprietary point correlation dimension algorithm (PD2i). The PD2i nonlinear algorithm is a deterministic, nonlinear measure of electrophysiological potentials to predict future pathological events.
Vicor currently has three products employing the PD2i nonlinear algorithm. The PD2i Analyzer, which has FDA 510(k) marketing clearance, measures heart rate variability. The physicians performing diagnostic tests with the PD2i Analyzer are able to receive reimbursement under existing CPT codes. The PD2i VS (Vital Sign), in clinical trials under a collaborative effort with the U.S. Army Institute for Surgical Research for risk stratification of combat and civilian trauma victims. The PD2i CA (Cardiac Analyzer) is in multiple clinical trials and identifies patients at risk of sudden cardiac death.
For more information: www.vicortech.com


 January 15, 2026
January 15, 2026 









