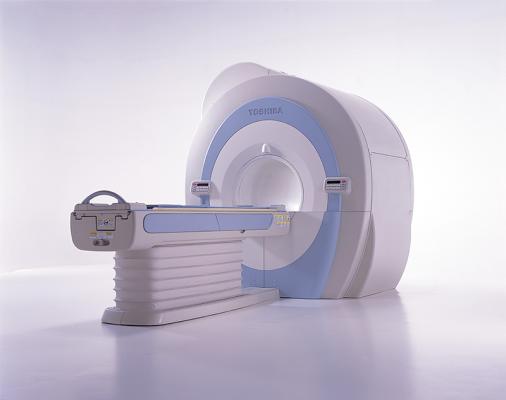
August 28, 2012 — Toshiba America Medical Systems Inc. has received U.S. Food and Drug Administration (FDA) clearance on its new Vantage Titan 1.5T series, which features the 8-, 16- and 32-channel MR (magnetic resonance) systems. The series offers a scalable solution with a full upgrade path from the 8-channel to the 32-channel system. In addition to these upgrades, the entire Titan 1.5T MR line is enhanced with a new modern, sleek exterior, including new covers and soft, patient-soothing lighting around the bore.
The Titan 8-channel is an ideal workhorse MR system for the majority of clinical applications, offering industry-recognized, patient-friendly features, including a 71-cm bore, Toshiba’s Pianissimo noise-reduction technology, advanced non-contrast imaging, integrated coils with Octave Speeder technology and an intuitive M-Power user interface.
For customers who require enhanced cardiac imaging, the Titan line is easily upgradeable to a 16- or 32-channel system, which will enable higher spatial and temporal resolution for better images of moving anatomy.
“With these new additions to the Titan 1.5T MR line, hospitals now have scalable MR technology options to meet a wider range of clinical needs,” said Stuart Clarkson, director, MR business unit, Toshiba. “Now, healthcare facilities can acquire an 8-channel system today with the knowledge that it can be upgraded to a high-end 1.5T MR system as a facility’s clinical applications expand and grow in the future.”
For more information: www.medical.toshiba.com


 January 21, 2026
January 21, 2026 








