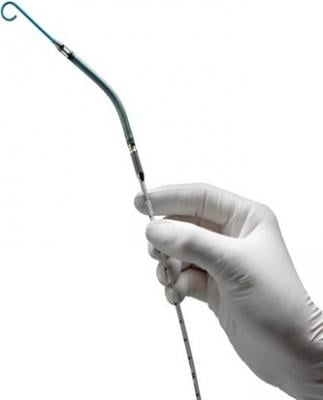
July 11, 2013 — Abiomed’s Impella percutaneous left ventricular assist device (pLVAD) was recently deployed at Baylor Medical Center in McKinney, Texas, to treat country music singer Randy Travis, said publicist Kirt Webster in a statement this week.
Travis was admitted July 7 with a “presumptive cardiomyopathy and congestive heart failure,” said William Gray, DO, FACC, director of cardiovascular services at Baylor Medical Center, in a video statement released on Baylor’s website.
Impella pumps are available in a variety of sizes and models. Delivered percutaneously during minimally invasive procedures, they deliver partial circulatory support for patients suffering from a range of heart problems. Though cost concerns remain, it is believed pLVADs may challenge intra-aortic balloon pumps (IABPs) when it comes to hemodynamic support.
The Impella LVAD was placed in the patient’s heart before he was transferred to The Baylor Heart Hospital in Plano on July 8. Since then he has suffered a stroke as a complication of the congestive heart failure, said Baylor physicians in a statement on the hospital’s website. He is currently in critical condition.
For more information: www.baylorhealth.com


 June 19, 2024
June 19, 2024 









