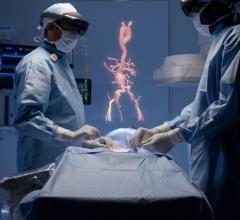
September 12, 2019 — HeartFlow Inc. has obtained clearance from the U.S. Food and Drug Administration (FDA) for the HeartFlow Planner, a non-invasive, real-time virtual modeling tool for coronary artery disease (CAD) intervention. The HeartFlow Planner will enable interventional cardiologists to virtually model clinical scenarios vessel-by-vessel, explore treatment strategies for patients with CAD before each procedure, review cases with colleagues and ensure everyone has a clear picture of the initial treatment plan.
“With the HeartFlow Planner, I am able to run multiple treatment scenarios in my office prior to the procedure, which enables me to plan ahead about the resources I will need in the catheterization lab,” said Mark J. Goodwin, M.D., interventional cardiologist, Advocate Health Midwest Heart Specialists and system director, Cardiac Innovations & Structural Heart Center, Edward-Elmhurst Health. “HeartFlow Planner is an intuitive consulting tool I can use to discuss cases with the entire heart team to ensure the team is prepared and engaged.”
“The information provided by the HeartFlow technology is powerful and will not only help us efficiently diagnose coronary artery disease but help us better understand different treatment options,” said Victor Marinescu, M.D., cardiologist, Advocate Health Midwest Heart Specialists and member of the medical staff, Edward-Elmhurst Health. “The visual nature of the HeartFlow Planner also makes it a great tool to explain to patients what will happen during their procedure.”
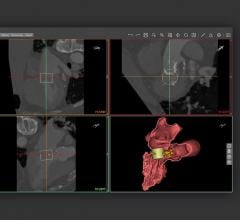
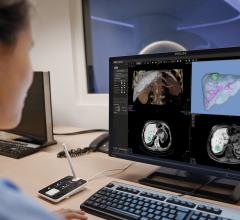
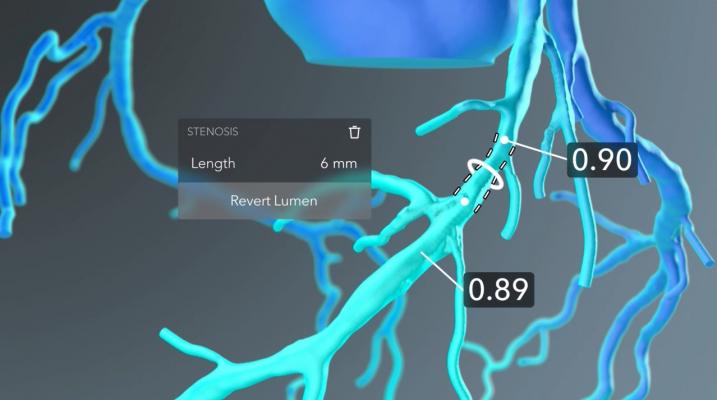
The HeartFlow Planner is a pre-procedure planning tool that is based on an idealized model of the HeartFlow Analysis, a color-coded 3-D model of a patient’s coronary arteries. Physicians can use this model to identify blockages and explore multiple treatment scenarios by virtually modifying the vessel. HeartFlow Planner will enable physicians to understand the impact of each modeled treatment strategy in real time.
Data from a patient’s non-invasive coronary computed tomography angiogram (CTA) are securely uploaded from the hospital’s system to HeartFlow’s software application running in the Amazon Web Services (AWS) cloud. HeartFlow leverages deep learning and highly trained analysts to create a personalized, digital 3-D model of the patient’s coronary arteries. The HeartFlow Analysis then uses computer algorithms to solve millions of complex equations to simulate blood flow and assess the impact of blockages on coronary blood flow. The HeartFlow Analysis is provided via a secure online interface to offer actionable information to enable clinicians to determine the optimal course of treatment.
The HeartFlow Planner utilizes an idealized coronary anatomy model and physiology simulation created from the HeartFlow Analysis. Physicians are able to identify stenoses and virtually modify the vessel. For each treatment scenario, HeartFlow Planner will display the modified fractional flow reserve-computed tomography (FFRct) values in real time to enable physicians to understand the impact of the modeled treatment strategy. Common scenarios for using HeartFlow Planner include focal stenoses, serial stenoses and borderline cases. HeartFlow Planner is only available on iOS platforms in the United States.
HeartFlow will be showcasing the HeartFlow Planner at the 2019 Transcatheter Cardiovascular Therapeutics (TCT) annual meeting, taking place in San Francisco, Sept. 25-29.
For more information: www.heartflow.com

 May 12, 2020
May 12, 2020