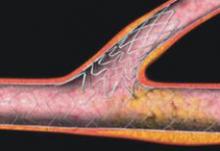
iffering approaches for the treatment of coronary bifurcation and ostial lesions (CBOL) continue to feed the debate over which stent designs and stenting techniques are most effective.
If you enjoy this content, please share it with a colleague
- Read more about Stents Side Tackle Bifurcations
- Log in or register to post comments