The EXCOR TAH-t has received CE approval with the CardioWest TAH-t. The pneumatic drivers provide precisely calibrated air pulses that make the world’s only FDA- and CE-approved TAH-t pump blood much like a human heart.
The EXCOR TAH-t portable driver is about the size of an attached case and weighs 20 pounds. It is designed for use by stable patients following TAH-t implant surgery. The portable driver allows stable patients to recover at home, which speeds recovery and lowers costs. The driver allows many patients to leave home to shop, visit friends and enjoy a fuller quality of life.
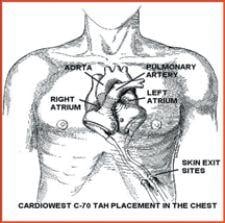
The TAH-t is a modern version of the Jarvik-7 Artificial Heart that was implanted in Barney Clark in 1982. It was used as a bridge to transplant for patients at risk of imminent death from irreversible biventricular heart failure. In the 1990s the device and technology moved to University Medical Center (UMC) in Tucson and was subsequently renamed the CardioWest temporary Total Artificial Heart.


 June 19, 2024
June 19, 2024 









