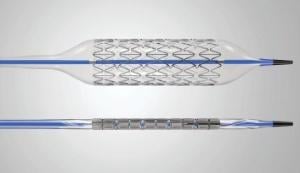
January 19, 2011 – The U.S. Food and Drug Administration (FDA) this week granted market clearance for Cook Medical’s Formula balloon-expandable renal stent system. The approval includes both the Formula 414RX Renal and the Formula 418 Renal stents.
FDA has said these devices are indicated for use in patients with atherosclerotic disease of the renal arteries following suboptimal percutaneous transluminal renal angioplasty (PTRA) of de novo or restenotic lesions.
The Formula 414RX Renal is a rapid exchange stent system with a 0.014-inch wire guide and the Formula 418 Renal is an over-the-wire system balloon with a 0.018-inch wire guide system. Both are engineered to cross lesions with high pushability, trackability and kink-resistance. Cook’s Formula stents are low-profile designs engineered to avoid shortening after deployment, allowing for precise placement.
“This approval greatly accelerates Cook Medical’s evolution as a leading provider of stents for the peripheral vessels,” explained Rob Lyles, vice president and global leader of Cook’s peripheral interventional business unit. “We are moving forward with important new products aimed at improving the physician’s ability to treat lesions with a new generation of stents and a dedicated suite of wires and balloons designed specifically for peripheral use.”
For more information: www.cookmedical.com


 November 24, 2025
November 24, 2025 









