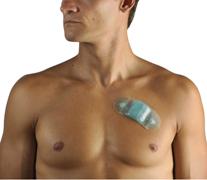
May 13, 2010 – An easy-to-wear, single-use, continuous ambulatory cardiac rhythm monitor yields high-quality diagnostic information, while providing greater patient comfort and improved compliance. The U.S. Food and Drug Administration (FDA) recently cleared the Zio Patch.
The companies announced today at the Heart Rhythm Society Scientific Sessions in Denver they entered into a partnership with a focus on iRhythm's Zio Patch. The companies said they are preparing for the product’s U.S. launch. The co-marketing partnership, which also includes iRhythm's Zio Event Card, a cardiac rhythm event monitor, will enable the rapid adoption of iRhythm's products and services by cardiologists and electrophysiologists.
"iRhythm aims to simplify the complexities of cardiac rhythm monitoring in order to streamline the referral process and promote timely access to specialty care and treatment," said Michael Rousseau, group president of St. Jude Medical. "Through this partnership with iRhythm, St. Jude Medical will provide physicians a way to more easily and cost-effectively triage and diagnose patients potentially experiencing arrhythmias."
Arrhythmias affect millions of Americans each year and left untreated, may lead to serious consequences, including stroke or sudden cardiac death. The new partnership brings together diagnostic devices and services to streamline the referral process, improve the patient experience, and promote timely access to specialty care and treatment.
For more information: www.sjm.com, www.irhythmtech.com


 January 15, 2026
January 15, 2026 









