September 8, 2014 — Eizo Inc. expanded its U.S. Food and Drug Administration (FDA) 510(k)-cleared large monitor system with the addition of the RadiForce LS580W monitor and LMM0802 large monitor manager for interventional radiology and surgical suites.
In 2011, Eizo received 510(k) clearance from the FDA for the RadiForce LS560W monitor and LMM56800 large monitor manager. With the introduction of the monitor’s successor model, the RadiForce LS580W and the LMM0802 large monitor manager as an alternative component, the company’s FDA-cleared large monitor system now offers additional options to medical professionals while continuing to provide a high level of customer assurance.
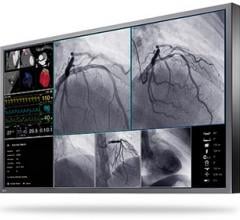
In the operating room (OR) and interventional suite, essential information such as X-ray, endoscopy and electrophysiology (EP) images is typically displayed using several different monitors. The RadiForce LS580W is a large 58-inch widescreen monitor with 4K ultra HD display for viewing a variety of essential medical information on a single screen. This allows users to view important information consistently and conveniently in one location. With the LMM0802 large monitor manager, users can scale and position several source video windows to suit their preferences and working conditions. This flexibility allows surgeons and interventionalists to easily optimize their workflow.
The FDA 510(k)-cleared RadiForce large monitor system consists of the combination of the following:
- One monitor selectable from these models: LS580W, LX600W, LX300W;
- One large monitor manager selectable from these models: LMM0802, LMM56800; and
- Accessories (optional): PDC0100 analog-DVI converter, PDS0800 DVI splitter/scaler, TDL3600 DVI transmission link, CID1000P touch panel console monitor.
For more information: www.eizo.com

 January 25, 2018
January 25, 2018