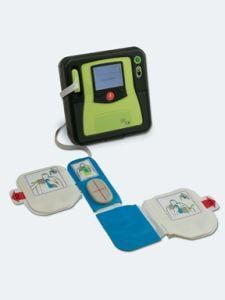
The FDA gave ZOLL Medical Corp. clearance to market and sell the ZOLL AED Pro with See-Thru CPR technology, which is designed to help minimize interruptions in CPR, as ZOLL aims to comply with one of the key recommendations of the American Heart Association’s (AHA) Guidelines for Advanced Cardiac Life Support.
See-Thru CPR is designed to reduce interruptions by allowing clinicians to see organized electrical activity during CPR compressions by filtering out compression artifact. This lets rescuers see a patient’s underlying cardiac rhythm during resuscitation efforts and eliminates the need to stop compressions to see if defibrillation was successful. See-Thru CPR is designed to automatically turn on when the rescuer switches the unit from semi-automatic to manual, which is accomplished by depressing two soft keys.

 April 02, 2019
April 02, 2019 



