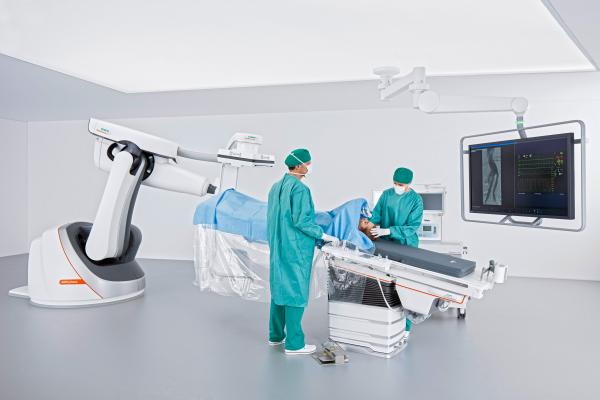
March 15, 2017 — The U.S. Food and Drug Administration (FDA) has cleared the Artis pheno robotic C-arm angiography system from Siemens Healthineers created for use in minimally invasive interventional procedures.
The Artis pheno possesses not only a zen40HDR flat panel detector and GIGALIX X-ray tube for high image quality, but also new 2k recording technology capable of delivering 2-D imaging resolution four times higher in all recording processes than its predecessor system, the Artis Zeego.
Siemens Healthineers designed the Artis pheno in part to assist hospitals in combating high rates of patient infection. An antimicrobial coating on the surface of the C-arm, stand and patient table may help prevent bacteria and viruses from multiplying on the system. The hermetically sealed housing of the C-arm and table modules, as well as easy-to-access spaces, aid in system cleaning. Additionally, the Artis pheno’s cabling is routed inside the system to prevent cables from becoming dirty and potentially transmitting bacteria. And since it is mounted on the floor rather than the ceiling, the system is not only easier to install in the operating suite, but the sterile air flow from the ceiling is uninterrupted.
The StructureScout exposure control feature can regulate acquisition parameters to automatically achieve optimal image contrast at ALARA (As Low As Reasonably Achievable) dose levels. And because the system also can scan up to 68 percent faster than its predecessor, its syngo DynaCT clinical software application can produce 3-D images that use less contrast media, increasing patient safety by decreasing the load on the kidneys.
The Artis pheno has a free inner diameter of 37.6 inches, which provides staff with enough room to remain at the side of pediatric patients. Bariatric patients are supported by the multi-tilt table, which can accommodate up to 617 pounds. And like the Artis zeego, the Artis pheno’s robotic construction provides a flexible isocenter, so it can follow all table positions while representing the patient’s target area from virtually any angle.
Interventionalists not only need to work easily while standing so they can perform lengthy procedures without fatigue, but they also must maintain optimal access within the operating area. Addressing these needs, the easy-float tabletop of the Artis pheno multi-tilt table can be moved with minimal effort, regardless of the tabletop’s angle or the patient‘s weight. The system recognizes the tabletop’s position at all times and automatically aligns to it. The memory positions allow the user to move the C-arm out of the operating area quickly and back to the same position for further imaging, so the user can perform a quality check during the operation.
The Artis pheno angiography system includes a broad array of software applications to aid users in performing a variety of interventional procedures:
- CLEARstent Live offers real-time verification of stent positioning during implantation;
- syngo CTO Guidance enables better clinician planning by delivering automatic segmentation of coronary computed tomography angiograms (cCTAs) as well as providing procedural guidance to avoid foreshortening;
- syngo DynaCT Cardiac permits 3-D visualization of a patient’s beating heart;
- syngo Aortic Valve Guidance delivers fast, precise 3-D information on aortic root anatomy for transcatheter aortic valve replacement (TAVR) procedures; and
- The syngo Fusion package superimposes previously obtained CT, magnetic resonance (MR) or positron emission tomography (PET)/CT images onto live fluoroscopy images.
For more information: www.usa.healthcare.siemens.com


 July 31, 2024
July 31, 2024 









