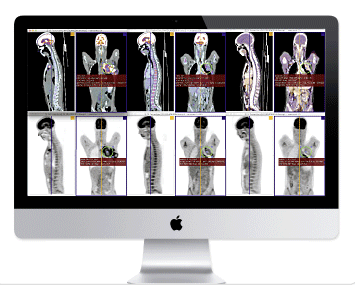
March 4, 2014 — Aycan and Chimaera GmbH received U.S. Food and Drug Administration (FDA) 510(k) clearance of Chimaera FusionSync, a software tool for more efficient handling and diagnosis of multi-modal and follow-up medical data. Available with Aycan workstation OsiriX Pro, Chimaera FusionSync was launched in Europe in 2013 with CE marking.
The Aycan workstation OsiriX Pro is an image-processing tool and DICOM PACS (picture archiving and communications system) workstation. The Chimaera FusionSync is an automatic image-registration algorithm. Together, they provide fast, reliable and automatic-rigid imaging to easily view multi-modal and follow-up series together for more efficient diagnoses. Users drag and drop two series together, then quickly navigate through large amounts of data for easy diagnosis based on spatial synchronization. Adaptions of view settings are completely eliminated, as all viewing properties are propagated to the linked series. Chimaera FusionSync currently supports 3-D computed tomography (CT), C-Arm CT, magnetic resonace imaging (MR) (including 2-D multi-slice MR), positron emission tomograophy (PET) and single positron emission computed tomography (SPECT) data, and is suited for oncology and other radiological applications where image comparison is vital.
Designed for post-processing and primary diagnosis, the 64-bit fast, Mac-based, vendor-neutral OsiriX Pro is an image-processing tool and DICOM PACS workstation for conventional, multi-slice and other image reading.
For more information: www.aycan.com

