
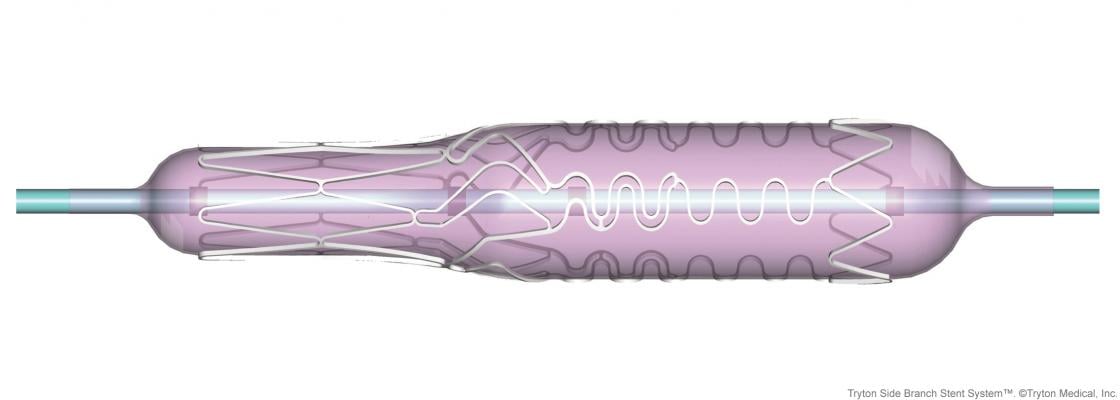
The Tryton Side Branch Stent is deployed in the side branch artery using a standard single wire balloon-expandable stent delivery system. A conventional drug eluting stent is then placed in the main vessel.

The Tryton stent delivery balloon.
March 6, 2017 – The U.S. Food and Drug Administration (FDA) has granted pre-market approval (PMA) for the Tryton Side Branch Stent for the treatment of coronary bifurcation lesions involving large side branches. It is the first dedicated bifurcation device to receive regulatory approval in the United States and addresses an unmet need in coronary interventions.
Watch a VIDEO demonstration of the deployment for the Tryton Dedicated Bifurcation Stent.
Coronary artery disease (CAD), the leading cause of death in the United States in both men and women, often results in the buildup of plaque at a site where one artery branches from another, also known as a bifurcation. Approximately 20-30 percent of all patients undergoing percutaneous coronary interventions (PCI) to open blocked arteries have a bifurcation lesion. Provisional stenting of the main branch is the current standard of care, but in many cases the side branch is not stented, leaving it vulnerable to complications like occlusion requiring bailout stenting.
“It is estimated that nearly a third of all patients treated with angioplasty each year have a bifurcation lesion, and the standard provisional treatment approach leads to side branch occlusion and bailout stenting far too often,” said Shawn McCarthy, president and CEO of Tryton Medical. “With this first-of-its-kind approval in the U.S., interventional cardiologists now have access to a stent that is specifically engineered to provide the complete lesion coverage and more predictable patient outcomes needed for the challenging anatomy of coronary bifurcation lesions.”
In a post hoc analysis of a randomized investigational device exemption (IDE) clinical trial, treatment with the Tryton Side Branch Stent in the intended population of patients with large side branches (appropriate for a ≥2.5 mm or larger stent) reduced the need for additional bailout stenting (0.7 vs. 5.6 percent, P = 0.02) and led to statistically significant lower side branch percent diameter stenosis at nine month follow up (30.4 vs. 40.6 percent, P = 0.004) when compared to provisional stenting. The post hoc analysis also showed comparable major adverse cardiovascular events (MACE) and myocardial infarction (MI) rates versus provisional stenting at three years.
The safety profile of the Tryton Side Branch Stent was validated in a confirmatory study that compared patients treated with the Tryton stent to a performance goal based on performance of the control arm from the randomized IDE clinical trial. The confirmatory study met its pre-specified primary endpoint, periprocedural myocardial infarction (PPMI), which was within its non-inferiority margin (Primary Endpoint: 10.5% + 95 percent C.I. vs. 17.9 percent, p=0.01).
“Treatment of complex lesions at the site of a bifurcation has historically been inconsistent, with results varying depending on the procedure and the experience of the interventionist,” said Aaron Kaplan, M.D., professor of medicine at Dartmouth Hitchcock Medical Center and chief medical officer of Tryton Medical. “A predictable bifurcation solution helps alleviate some of the stress in these procedures by limiting variability and reducing the need for bailout stenting. This important FDA decision could have a profound impact on treatment protocols and guidelines for significant bifurcation lesions in the years ahead.”
Tryton has signed a strategic distribution agreement with Cardinal Health enabling Cordis, its interventional vascular business, to be the exclusive distributor of the Tryton Side Branch Stent in the U.S. “This strategic agreement is a significant step for Cordis to quickly get innovative technologies into the hands of our customers in the U.S. We are actively preparing to commercially launch this product with Tryton to ensure physicians will soon have a new treatment option in their cath labs to help deliver the best patient care available,” said David Wilson, president of Cordis.
The Tryton Side Branch Stent is available in multiple device diameters (2.5 to 3.5 mm in the side branch) and is compatible with any conventional drug eluting stent in the main vessel.
The stent is built using proprietary Tri-ZONE technology to offer a dedicated strategy for treating bifurcation lesions. Tryton’s cobalt chromium stent is deployed in the side branch artery using a standard single wire balloon-expandable stent delivery system. A conventional drug eluting stent is then placed in the main vessel. The Tryton Side Branch Stent has now been used to treat more than 12,000 patients worldwide. It is commercially available in multiple countries within Europe, the Middle East and Africa, and is now approved for use in the U.S.
Links to more Information on the Tryton Sidebranch Stent:
Tryton Side Branch Stent Meets Primary Endpoint in Pivotal Confirmatory Study
New Designs May Ease Bifurcation Stenting
New Directions for Stent Technology
For more information: www.trytonmedical.com


 July 31, 2024
July 31, 2024 









