September 26, 2019 — The U.S. Food and Drug Administration (FDA) has cleared three modules of AI-Rad Companion Chest CT, an intelligent software assistant from Siemens Healthineers that brings artificial intelligence (AI) to computed tomography (CT). They include the AI-Rad Companion Engine (K183272), Pulmonary (K183271) and Cardiovascular (K183268) modules. Representing the first intelligent assistant of the new AI-Rad Companion platform, AI-Rad Companion Chest CT helps radiologists interpret images of the thorax (chest) quickly with desired accuracy and precision, and automatically documents these findings as structured reports.
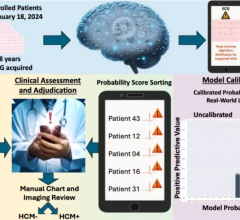
Although CT chest images are mainly assessed by radiologists with regard to the primary indication, these images contain additional clinically relevant information. The algorithms in AI-Rad Companion Chest CT were trained on extensive datasets and annotated by qualified clinical specialists to provide segmentation, measurement and highlighting of key anatomical structures, to support quantitative and qualitative analysis.
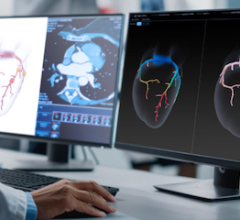
Using CT images of the chest, AI-Rad Companion Chest CT differentiates among various structures in that region – including the lungs, heart and aorta – highlights them individually, and marks and measures potential abnormalities, such as coronary calcifications. It supports a variety of tasks, including:
-
Automated detection of lesions, localization of abnormalities and measurement of lung lesions;
-
Quantification of per-lobe low-attenuation parenchyma;
-
Enhanced visualization of lung lesions;
-
Automated segmentation of lung lobes and enhanced visualization of low-attenuation parenchyma;
-
Segmentation and measurement of maximum diameters of the thoracic aorta;
-
Quantification of the total calcium volume in the coronary arteries; and
-
Detection of nine anatomical landmarks as identified by American Heart Association (AHA) guidelines.
Based on the AI-supported analysis, AI-Rad Companion Chest CT automatically generates standardized, reproducible, and quantitative reports in Digital Imaging and Communications in Medicine (DICOM) SC format. In addition to reducing time spent on manual results documentation, these reports can be accessed by radiologists on the picture archiving and communication system (PACS) in the clinical routine. AI-Rad Companion Chest CT also highlights potentially clinically relevant changes that might otherwise remain unnoticed because they were not the primary indication for the exam.
AI-Rad Companion Chest CT is a cloud-based solution that has been tested and validated for CT scanners from Siemens Healthineers, GE Healthcare and Philips Healthcare. It uses certified, secure teamplay infrastructure and integrates seamlessly into existing clinical workflows.
For more information: www.siemens-healthineers.com


 September 24, 2025
September 24, 2025