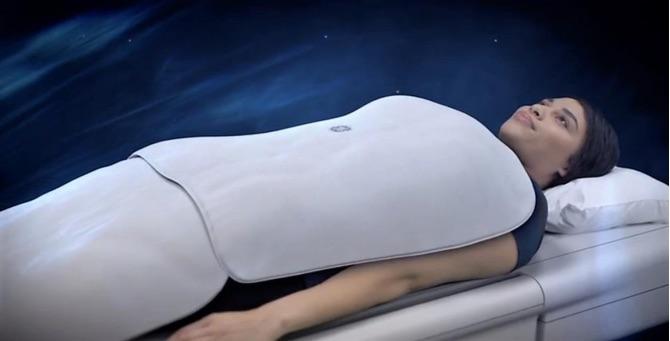
December 13, 2017 — GE Healthcare announced U.S. FDA 510(k) clearance of its new Air Technology, what it calls an industry-first suite of radiofrequency (RF) coils that enables total freedom in coil positioning and handling during a magnetic resonance imaging (MRI) scan. The new coil design is 60 percent lighter than conventional coils, benefiting both patients and technologists, offers greater flexibility in all axes to help conform to patients’ anatomies and fits all patient ages, sizes and shapes.
Air Technology uses a flexible conductor material that allows each coil element to be closer to the anatomy to improve signal reception, depth of penetration and image quality. Its ultra-lightweight and flexible design makes it easier for the technologist to position the coil on the patient.
“In my opinion, one of the greatest advancements in MR is Air Coil Technology,” said Tammy Heydle, senior MR technologist at the University of Wisconsin – Madison and part of the team who evaluated the Air Technology Suite in a clinical study. “There are almost endless possibilities for its use. The Air Coils are light like a blanket, versatile and very comfortable for patient positioning.”
The unique electrical properties of Air Technology also address clinical productivity by minimizing coil coupling between elements and enabling arrays with higher element density and more robust parallel imaging with higher acceleration factors.
“I’m pleased with the image quality,” said Scott Reeder, professor of radiology, chief of MRI, vice chair of research at University of Wisconsin – Madison, who led the clinical study. “The coils have great signal- to-noise-ratio. And our patients have given us excellent feedback on the overall comfort of the coil.”
By avoiding the copper etching of elements in traditional coil manufacturing, flexible Air Coils are constructed using a sustainable manufacturing process that is up to 90 percent greener through a reduction of typical electronics and packaging waste. Air Technology also consumes 50 percent less power compared to conventional coils.
Air Technology is currently available on Signa Premier, GE Healthcare’s newest wide bore 3.0T MRI system. It is available as a 48-channel head coil designed to fit 99.9 percent of patients, a 30-channel anterior array providing 65 cm of coverage and a 60-channel posterior array providing 110 cm of coverage.
For more information: www.gehealthcare.com


 January 21, 2026
January 21, 2026 








