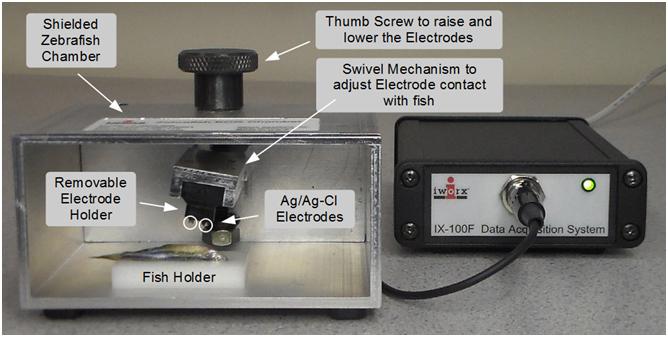
August 13, 2015 — iWorx Systems Inc., developer of advanced physiology teaching and research instruments and software, has introduced a compact, non-invasive system (patent pending) for recording and analyzing electrocardiogram (ECG) data from zebrafish.
The iWorx ZS-200 Zebrafish ECG System includes a high-resolution, low-noise single-channel recorder optimized for recording microvolt ECG signals. The system also comes complete with a recording chamber, fish pedestal, Ag/AgCl electrodes, cable assembly and iWorx LabScribe3 recording software with the ECG Analysis Module.
iWorx LabScribe ECG Analysis Module automates the analysis of ECG data. Specific analysis templates are included to accurately delineate PQRST onset, durations and amplitudes. Custom templates can also be created based on unique ECG profiles and saved to a library for future use.
Other features include beat averaging, beat classification, outlier removal, Pointcaré plots, scattergrams and the ability to easily extract source data and average data as images or text files. Also included is a comprehensive list of 24 calculations including R-R, PR, QT, QR and QTc intervals, QRS, T, P and TP durations, P, Q, R, S, T amplitudes and ST elevation.
For more information: www.iworx.com


 January 15, 2026
January 15, 2026 









