October 26, 2011 – Lantheus Medical Imaging announced it has been awarded three-year supplier contracts with Premier Purchasing Partners, the group purchasing unit of the Premier healthcare alliance, in the category of cardiac ultrasound contrast media for its ultrasound contrast agent, Definity Vial for (perflutren lipid microsphere) Injectable Suspension, and its magnetic resonance angiography (MRA) blood pool imaging agent, Ablavar (gadofosveset trisodium).
The cardiac ultrasound contrast media contract award is a three-year sole-source supplier agreement, effective Dec. 1, 2011 through Nov. 30, 2014. The Ablavar contract award is a three-year supplier agreement, effective Jan. 1, 2012, through Dec. 31, 2014. These new agreements allow Premier members, at their discretion, to take advantage of special pricing and terms pre-negotiated by Premier for Definity and Ablavar .
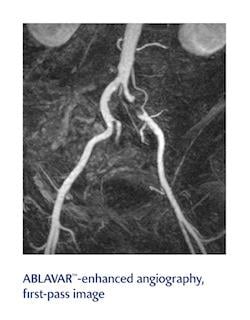
Definity, an ultrasound contrast agent for use in patients with suboptimal echocardiograms to opacify the left ventricular chamber and to improve the delineation of the left ventricular endocardial border cardiograms, and Ablavar, an MRA contrast agent indicated to evaluate aortoiliac occlusive disease (AIOD) in adults with known or suspected peripheral vascular disease (PVD), will both be offered to more than 2,500 hospitals nationwide and 76,000-plus healthcare sites. The contracts were awarded after a competitive bidding process that examined multiple factors, including pricing and clinical experience with Definity and Ablavar, as well as Lantheus’ support of the products.
For more information: www.lantheus.com, www.ablavar.com

 October 09, 2025
October 09, 2025