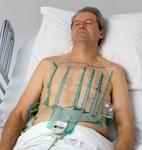
May 29, 2009 - Saint Francis Hospital in Evanston, Ill. has become the first hospital in Illinois to offer the new PRIME ECG made by Heartscape Technologies, which has a disposable vest containing 80 electrocardiogram leads that detect heart rhythm and blockages in 360-degrees of the heart.
The new vest is expected to help identify heart attacks that might otherwise go undiagnosed. Traditional ECG tests contain only 12-leads that are applied only to the front of a patient's torso and can not monitor electrical impulses in the back or side areas of the heart, which may have undetected blockages and may be the source of chest pain. With the new ECG test, in 10 minutes or less, physicians will know more about the patient and the patient's heart condition than ever before, the hospital said.
PRIME ECG provides physicians with a full 360-degree view of the heart's arteries, without surgical intervention, and can more rapidly and accurately identify closed arteries that might go unnoticed using traditional ECG equipment. Clinical research has shown that the use of large numbers of electrode sites about the front, sides and posterior of the torso provides valuable diagnostic information that can lead to earlier diagnosis for many acute coronary syndrome (ACS) patients. This procedure has traditionally been called body surface mapping (BSM).
"The 80-lead ECG is the next step in advancing emergency cardiac care. Traditional tests do not detect all heart attacks or heart muscle damage or blockages in all patients. This test is an additional safeguard that can be used on patients experiencing chest pain who have tests that are otherwise normal," said Shahriar Dadkhah, M.D., FACC, cardiologist and director of cardiology research at Saint Francis Hospital.
Dr. Dadkhan said the test can catch about 20 out 100 silent heart attacks, which would otherwise go undetected with 12-lead ECG equipment.
For more information: www.heartscape.com


 January 15, 2026
January 15, 2026 









