December 20, 2012 — At the 98th annual meeting of the Radiological Society of North America (RSNA), Royal Philips Electronics continued the Imaging 2.0 journey by showcasing several new features for existing image modalities that deliver clinical benefits to customers while simultaneously answering the economic challenges clinicians face worldwide.
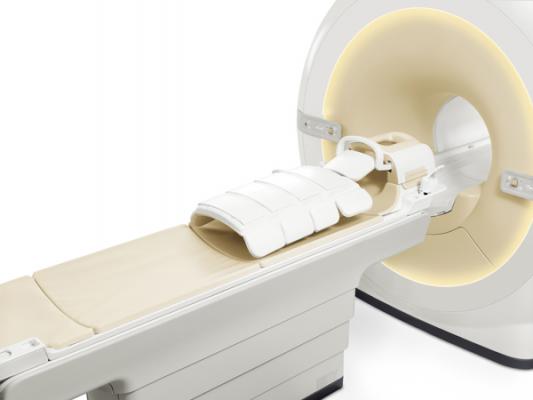
At RSNA, Philips displayed dStream broadband technology that will enable current Philips MR users to switch to digital broadband MRI (magnetic resonance imaging) for the majority of analogue Philips MR systems. The technology builds on the existing MR magnet and is a cost-effective way to provide digital broadband MRI. Compared with system replacement, dStream upgrade technology saves on magnet and reconstruction costs and means fewer disturbances for the facility during installation. Users can expect significant savings in cost when upgrading an existing MRI compared to purchasing a new digital system. Philips introduced the dStream technology with its Ingenia 1.5T and 3.0T MR systems.
“We know that customers around the globe are facing economic struggles and purchasing decisions may be uncertain,” said Gene Saragnese, CEO of imaging systems at Philips. “Philips understands these challenges and is committed to continually improving access to the best care possible and helping clinicians to improve patient outcomes. We do this by providing upgrades and enhancements that meet these challenges, which allows our customers to improve on the existing imaging system investments they have already made.”
SmartPath to dStream features signal digitization directly at the patient, delivering high signal-to-noise ratio (SNR) that benefits image quality and speed. The lightweight dStream digital coils are comfortable for patients and the easy coil handling significantly benefits workflow. SmartSelect automatically determines the coil elements to use, shortening scan setup time.
With the addition of dStream technology to an MRI system, routine exams for brain, spine, knee, ankle and liver can now be performed in less than eight minutes. The technology digitizes the MR signal at its purest spot in the RF coil, increasing SNR and allowing for enhanced image quality. Fewer coils lead to reduced coil positioning and patient setup time.
Additionally, Philips is now offering the addition of transducers for the surgical suite on Philips’ CX50 compact ultrasound system. The most challenging cases for clinicians often occur in the ICU or the surgical suite, where space is at a premium. The addition of this strategic upgrade would bring premium, mobile ultrasound imaging to surgical procedures.
For more information: www.philips.com/RSNA


 January 21, 2026
January 21, 2026 








