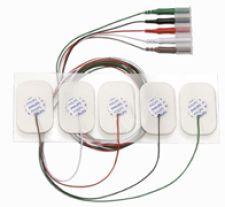
Philips offered new disposable adult ECG leadwire electrodes to help with infection control May 3-8 at the American Association of Critical-Care Nurses National Teaching Institute in Chicago.
Philips said about 1.7 million hospital-acquired infections are reported each year in U.S. hospitals, and drug-resistant pathogens can live on surfaces in hospitals, such as ECG lines, for weeks. The new disposable leadwire electrodes are designed to be a part of a hospital’s HAI prevention strategy.
Available in three or five-lead configurations with metallic radiolucent wires, the leadwire electrodes are available with AAMI and IEC color code wires for faster lead placement. Philips said the sold-get electrode technology has an estimated 72-hour wear time and can be repositioned one time. The electrodes do not need an adaptor, as they plug directly into the Philips trunk cable.
May 2008


 January 15, 2026
January 15, 2026 









