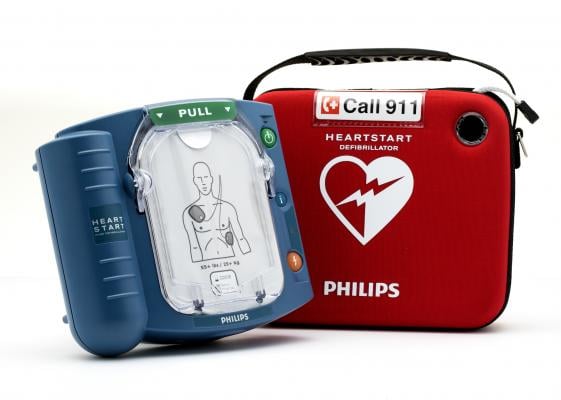
The Philips HeartStart OnSite automated external defibrillator. Image courtesy of Philips Healthcare.

The Philips HeartStart Home automated external defibrillator. Image courtesy of Philips Healthcare.
June 19, 2019 — Philips announced the U.S. Food and Drug Administration (FDA) has approved the company’s premarket approval (PMA) application for its HeartStart OnSite defibrillator (model M5066A) and HeartStart Home defibrillator (model M5068A). The approval includes the relevant supporting accessories, such as batteries and pad electrodes.
The HeartStart OnSite and HeartStart Home defibrillators are the only over-the-counter automated external defibrillators (AEDs) available to consumers in the U.S., according to Philips. The company also said the HeartStart Home defibrillator is the only AED specifically indicated for home environments.
The PMA application for Philips’ HeartStart OnSite and HeartStart Home defibrillators can be found here. These devices already had FDA 510(k) clearance, but are now subject to PMA.
For more information: www.usa.philips.com/healthcare


 January 13, 2026
January 13, 2026