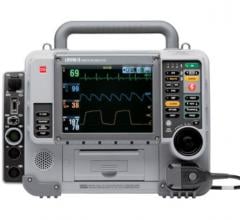
September 25, 2013 — Physio-Control launched its comprehensive solution designed to improve cardiac resuscitation in hospitals. The CodeManagement Module, which adds capnography and wireless data capabilities to the Lifepak 20/20e defibrillator/monitor platform, received 510(k) clearance from the U.S. Food and Drug Administration (FDA) in August and achieved the CE mark for commercialization worldwide in April.
The CodeManagement Module delivers the ability to move event and device data via the Lifenet System data network into Code-Stat Data Review Software, providing hospital care teams with tools to measure system quality and review cardiac resuscitation performance. These tools were developed in response to the 2010 American Heart Assn. (AHA) and European Resuscitation Council (ERC) Guidelines and in anticipation of the 2013 AHA Consensus Statement, “CPR Quality: Improving Cardiac Resuscitation Outcomes Both Inside and Outside the Hospital.”[1] The module also adds capnography, a vital tool for monitoring cardiopulmonary resuscitation (CPR) effectiveness, endotracheal tube placement and return of spontaneous circulation (ROSC). Additional features of this new release include:
- Lifenet Asset — allows remote access to Lifepak devices to monitor battery charge, update software and view self-test status
- Lifepak 20/20e metronome — helps rescuers perform chest compressions at the AHA/ERC Guideline recommended rate
- Larger code management clock — a 70-percent larger clock on the 20/20e provides better visibility by the entire code response team and a centralized source for time management and documentation
The CodeManagement Module is an integral part of the Physio-Control System of Care, a customized combination of lifesaving hardware, software and services designed to work together or independently, offering customers a comprehensive resuscitation solution to meet their unique requirements and protocols. The CodeManagement Module can be added to a hospital’s existing Lifepak 20/20e devices or can be purchased with new device deployments. In addition to the CodeManagement Module, the Physio-Control System of Care is comprised of:
- Lifepak monitor/defibrillators — hospitals can choose between the simplicity of the 20e, the ruggedness and flexibility of the Lifepak 15 with waveform capnography or the Lifepak CR Plus or Lifepak 1000 AEDs for lower acuity areas
- Lifenet System — a Web-based data network and suite of tools to speed time-to-treatment, measure performance, trim operational costs and streamline equipment management
- Code-Stat Data Review Software with Advanced CPR Analytics — captures chest compression, shock delivery and other event data from all Lifepak devices for post-event review and quality improvement
- TrueCPR coaching device — simple-to-use tool that accurately measures manual chest compressions to optimize manual CPR by providing high-quality feedback, in both real-time and following a resuscitation event, using new technology that has been shown to provide accurate depth measurement
- LUCAS Chest Compression System — Physio-Control’s mechanical CPR solution; easy to apply, provides consistent compressions of at least 100 compressions/minute and 2 inches (5 centimeters) of depth, with minimal interruptions, even during patient transport or in the catherization lab
For more information: www.physio-control.com
1. Meaney PA, Bentley BJ, Mancini ME, et al. CPR Quality: Improving Cardiac Resuscitation Outcomes Both Inside and Outside the Hospital: A Consensus Statement From the American Heart Association. Circulation. June 2013; 0009-7322:1-20.


 January 13, 2026
January 13, 2026