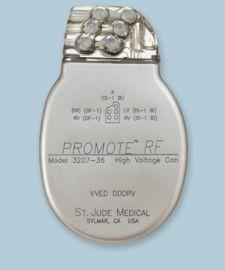
St. Jude Medical Inc. announced FDA approval of its first radiofrequency (RF) wireless devices to treat patients with heart failure and with potentially lethal heart arrhythmias. The Promote RF CRT-D (cardiac resynchronization therapy defibrillator) and Current RF ICD (implantable cardioverter defibrillator) feature radiofrequency telemetry for wandless communication with programmers used by physicians to interrogate and program devices.
The Promote RF CRT-D reportedly allows physicians to electronically reconfigure left ventricular (LV) leads to help optimize the pacing performance of the device without the need to physically reposition the lead. The devices also feature the VIP (Ventricular Intrinsic Preference) algorithm that is designed to allow the patient’s own heart rhythm to prevail when possible. VIP technology actively monitors the heart on a beat-by-beat basis to provide pacing only when needed, which has been shown to be better for patients’ overall heart health.


 January 13, 2026
January 13, 2026