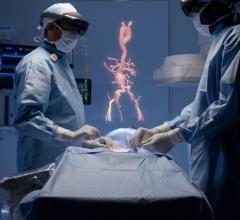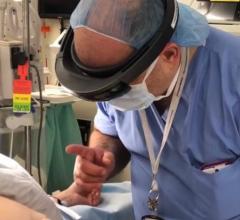
November 30, 2012 — TeraRecon previewed a flexible new pay-as-you-go billing option for its cloud and laptop users of its iNtuition advanced visualization tools at the 39th Annual Veith Symposium in New York Nov. 14 – 16, 2012. The software supports physicians involved in TAVI/TAVR, EVAR and TEVAR.
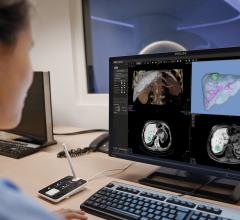
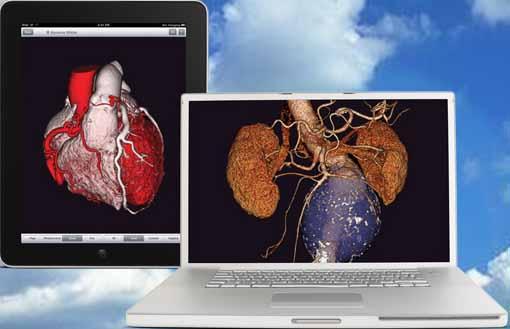
iNtuition is available both as a laptop-based software solution (no requirement for internet connection), and as an internet-based service, iNtuition Cloud supporting secure upload of image data and real-time interactive use of the full suite of advanced visualization tools, through a web browser.
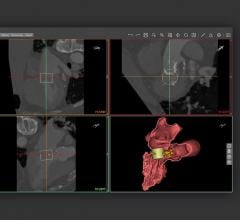
Traditionally, sophisticated advanced visualization of computed tomography (CT) and magnetic resonance imaging (MR) images has required a costly dedicated computer workstation, but with iNtuition a powerful suite of visualization tools may be run on a laptop computer, or via the Internet using TeraRecon’s secure and HIPAA-compliant iNtuition Cloud service.
For Veith 2012, TeraRecon will preview a new flexible billing option, where iNtuition subscribers will be billed only for the studies processed and/or stored with the TeraRecon software, either locally on a laptop-based installation, or on iNtuition Cloud.
“The traditional commercial model of advanced visualization works well for busy departments with a predictable volume of scans, but it denies affordable access to these valuable tools, by the physicians who process only a few cases a month, and who need to match the expense of 3-D software with the unpredictable economics relating to the procedures actually performed,” said Robert Taylor, Ph.D., TeraRecon president and CEO. “This new billing option will overcome this obstacle by enabling physicians to access the software initially for free, for a limited number of cases, after which they can obtain more credits from TeraRecon, or from other sources such as device companies, or be billed on a monthly basis depending on usage. We are excited about the potential of this new payment model, to greatly improve the accessibility and affordability of advanced processing tools.”
For more information: www.terarecon.com

 May 12, 2020
May 12, 2020