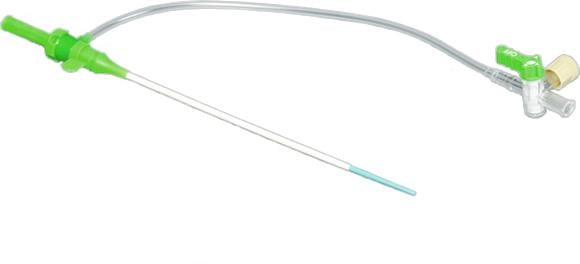
November 1, 2012 — Terumo Interventional Systems announced the nationwide availability of its new Pinnacle Precision Access System. The vascular access system specifically designed for smooth, efficient and reliable peripheral vascular access. It offers the only available tapered introducer needle to minimize vessel trauma, dilate the tissue and gain more precise access in difficult cases, such as those impeded by scar tissue or calcification.
“Terumo’s Pinnacle Precision Access System is one of the most innovative advancements in technology that will help us achieve success in peripheral vascular intervention, specifically where critical limb ischemia (CLI) is present,” said Jihad Mustapha, M.D., FACC, FSCAI, director of endovascular laboratories and director of cardiovascular research at Metro Heart and Vascular, Metro Health Hospital, in Wyoming, Mich. “From the tapered needle to the TIF (total integrated fit) sheath, Terumo provides a seamless transition for vascular access femorally or peripherally.”
One of the system’s most significant advantages is the elimination of procedural steps compared to alternative products. Traditionally, once access is achieved, the site is gradually enlarged to the desired French size by exchanging to a larger wire and sheath. Terumo’s new precision access system eliminates the need for both a micropuncture kit and a standard size introducer sheath. It features proven TIF technology and a new tapered 21 gauge needle that allow for a smaller, straighter and more accurate puncture to significantly reduce vessel entry site trauma and access complications.
The system was designed and developed at Terumo’s manufacturing facility in Elkton, Md. The upgraded facility combines manufacturing and new product development teams in the same geographic location to streamline research and development while maximizing manufacturing efficiencies.
For more information: www.terumois.com


 September 12, 2025
September 12, 2025 









