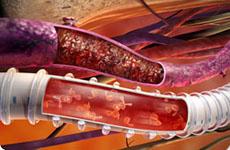
December 21, 2009 – The recently introduced GORE PROPATEN Vascular Graft has integrated rings that reduce the cross-sectional profile by 24 percent as compared to externally ringed grafts, and its radial reinforcement allows for improved blood flow. GORE introduced the new Vascular Graft at the 2009 AIMsymposium/VEITHsymposium in November in New York City. The grafts offer radial support that is concealed within the graft wall and resists kinking and compression. The stent graft also has a covalently-bonded PROPATEN Bioactive Surface to help prevent thrombus formation. The coating uses heparin on the luminal surface, which allows the graft to retain its thromboresistant bioactive properties over time. “Optimal graft performance in vascular surgery requires materials to be flexible to allow for smooth implantation,” said Michael Stoner, M.D., associate professor of vascular surgery for the East Carolina Heart Institute at East Carolina University, Greenville, N.C. “The new configurations of the GORE PROPATEN Vascular Graft with Integrated Rings will allow for easier placement through a tissue tunnel and enable clinicians to cut and sew through the radial support anywhere along the entire length of the graft. Based on experience to date, I am optimistic that this new feature will further help surgeons more effectively treat challenging cases and improve the outcomes of patients with vascular disease.” The manufacturer says the GORE PROPATEN Vascular Graft is the first vascular graft approved for hemodialysis access and the treatment of peripheral artery disease (PAD). For more information: www.goremedical.com


 September 12, 2025
September 12, 2025 









