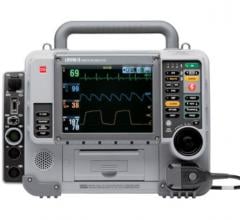
Zoll’s RescueNet 12-Lead is a free and versatile transmission system that allows healthcare providers to receive and manage 12-lead ECGs from just about anywhere. It is browser based for maximum flexibility and universal access. RescueNet 12-lead data can be accessed via e-mail, fax, computer, tablet, or handheld simultaneously. The system is safe and effective for hospitals. All information is encrypted and password protected, and supports customer compliance requirements with HIPAA and HITECH. All 12-leads are archived in the cloud and system activity reports provide information to manage workflow.
For more information: www.zoll.com


 January 13, 2026
January 13, 2026