June 15, 2012 — At the Society of Nuclear Medicine’s (SNM) 59th Annual Meeting in Miami Beach, Fla., June 9-12, Cardinal Health introduced new technology enhancements that make it possible for hospital nuclear medicine departments and imaging centers to seamlessly improve data integration and the overall efficiency of their operations.
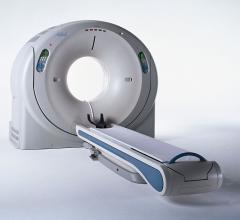
June 15, 2012 — To enable hospitals to keep pace with the latest technological advances and offer better patient care and improved workflow while containing costs, Toshiba America Medical Systems, Inc. announces the availability of VeloCT.
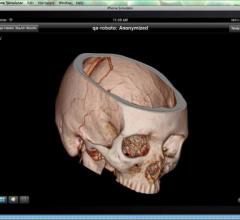
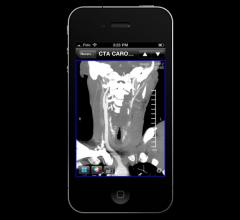
June 14, 2012 — Fujifilm Medical Systems USA Inc. announced the availability of Synapse Mobility 3.0, a new zero-footprint application with improved features that enable access to Fujifilm's suite of Synapse products, from handheld mobile devices to Macintosh- or Windows-based PCs.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

June 14, 2012 — A U.S. Food and Drug Administration (FDA) advisory panel voted in favor of recommending expanding the indication for the Edwards Lifesciences Sapien transcatheter heart valve for the treatment of high-risk patients. Currently, the Sapien is only indicated for patients who are too sick to undergo valve replacement surgery for severe, symptomatic aortic stenosis. The panel voted 11-0, with one abstention, that the benefits of the heart valve outweighed the risks for these patients.
June 14, 2012 — Abiomed Inc. said today it received Health Canada approval to market the Impella cVAD (ventricular assist device), a new percutaneous Impella heart pump that provides peak flow of approximately 4 liters of blood per minute.
June 13, 2012 — Physicians presented at EuroPCR 2012 the results of two multicenter, randomized controlled trials, the BELLO and PACIFIER studies, each one showing statistically significant advantages of using an IN.PACT drug-eluting balloon from Medtronic Inc. over a corresponding conventional treatment for coronary and peripheral artery disease (CAD and PAD), respectively.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
June 13, 2012 — AliveCor announced it has raised $10.5 million in Series B venture financing. The financing comes on the heels of recent presentations at major cardiology conferences, in which supporting data demonstrated the clinical utility and accuracy of AliveCor's device in monitoring patients' heart health.
June 13, 2012 — Positron Corp. announced completion of its first strontium validation effort in May 2012, when Manhattan Isotope Technology (MIT), a Positron subsidiary, successfully processed strontium.
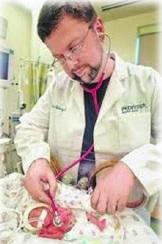
As the nation’s leading provider of maternal-fetal, newborn and pediatric subspecialty physician services, Pediatrix Medical Group says streamlining the transfer of images and reports is a priority. Seeking a faster, more reliable method of exchanging files than on CD, the group adopted a cloud-based medical information exchange.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Best Cyclotron Systems is unveiling five new cyclotrons for isotope research and production at the Society of Nuclear Medicine (SNM) 2012 Annual Meeting on June 9-12 in Miami Beach, Fla.
Boston Scientific Corporation has closed its acquisition of Cameron Health and, as a result, added to its product portfolio the world's first and only commercially available subcutaneous implantable cardioverter defibrillator, called the S-ICD System.
June 11, 2012 — Boehringer Ingelheim Pharmaceuticals Inc. announced that the Pradaxa (dabigatran etexilate mesylate) capsules prescribing information has been updated to affirm that "Pradaxa 150 mg twice daily was superior in reducing ischemic and hemorrhagic strokes relative to warfarin."
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
June 8, 2012 — Bayer HealthCare announced the formation of a strategic partnership with Radimetrics Inc. that will offer U.S. healthcare professionals the industry’s first computed tomography (CT) dose management software portfolio to include radiation and contrast dose tracking. Through the agreement, Bayer will distribute the Radimetrics eXposure Radiation Dose Management (RDM) software in addition to Bayer’s existing contrast-focused dose management solution.
June 11, 2012 — Critical Diagnostics, a U.S.-based biomarker company focused on cardiovascular diseases, and Health Diagnostic Laboratory Inc. (HDL) announced an agreement under which HDL will soon offer ST2 testing services based on Critical Diagnostics' Presage ST2 Assay.
June 11, 2012 — Calgary Scientific Inc. has received clearance from the U.S. Food and Drug Administration (FDA) to market its ResolutionMD Web 2.9 Vessel Analysis module for post-processing diagnostic review and analysis of patient computed tomography (CT) and magnetic resonance imaging (MRI) scans.

 June 15, 2012
June 15, 2012