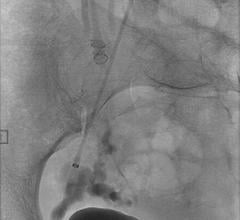
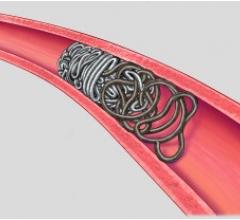
April 12, 2012 – Terumo Interventional Systems recently announced the nationwide availability of the Azur D35 Framing Coil, the first truly detachable coil that precisely frames the targeted area for peripheral embolization.
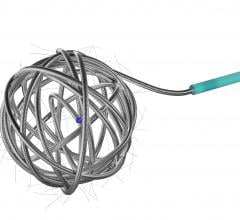
The new Azur D35 Framing Coil is indicated for peripheral embolization procedures where a scaffolding structure is needed, such as: hypogastric artery embolization; peripheral arteriovenous malformations and fistulas; endoleaks; visceral aneurysms; and venous applications. The detachable embolic platform forms a spherical 3-dimensional shape when deployed and is designed to control the first coil deployment, when a standard coil would not suffice due to high flow and high pressures.
“The new Azur D35 Framing Coils’ innovative technology offers interventional radiologists and vascular surgeons options in challenging, high-flow procedures,” said Chris Pearson, vice president, marketing, Terumo Interventional Systems. “These detachable framing coils afford greater filling capacity, packing density and mechanical occlusion, particularly in the treatment of high-flow
and challenging blood vessels, vascular malformations and aneurysms.”
According to Pearson, the Azur D35 Framing Coil offers enhanced delivery control that allows for precise placement prior to final deployment.
“The detachable technology allows the physician to optimize the framing coil placement, creating support for subsequent coils,” said Pearson. “Azur D35 Hydrogel coils expand gradually upon exposure to blood, which provides improved volumetric filling allowing highly effective, targeted embolization. Physicians now have a complete coil embolization solution from Terumo.”
For more information: www.terumois.com


 June 05, 2025
June 05, 2025