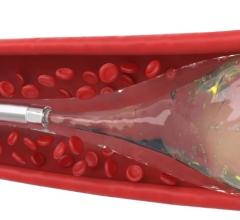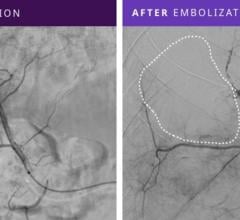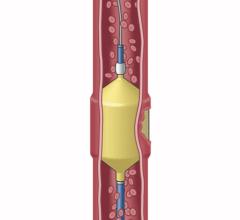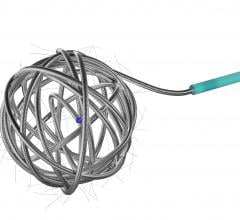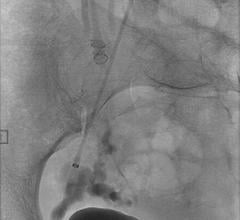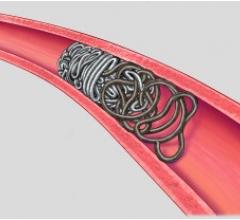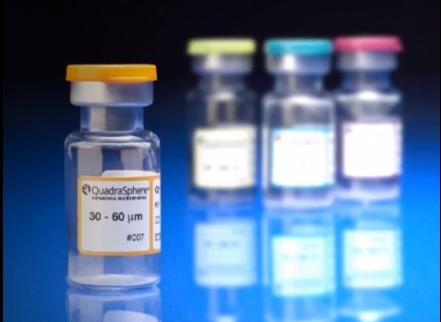
Image courtesy of Merit Medical Systems Inc.
August 17, 2015 — Merit Medical Systems Inc. announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) expanding indication for QuadraSphere Microspheres to include the embolization of hepatoma. Malignant hepatoma, also known as hepatocellular carcinoma (HCC), is the sixth most common cancer and the third leading cause of cancer deaths worldwide. QuadraSphere Microspheres are precisely calibrated and designed to offer controlled, targeted embolization, treating HCC by stopping blood flow to the tumors.
For more information: www.merit.com


 June 05, 2025
June 05, 2025