January 14, 2016 — Penumbra Inc. announced the U.S. launch of its new POD Packing Coil, designed as a complementary device for Penumbra’s Ruby and POD (Penumbra Occlusion Device) embolization products. This latest launch adds to the company’s rapidly expanding peripheral vascular product portfolio.
Nearly 900,000 Americans each year suffer from peripheral vascular conditions involving acute clots or aneurysms that occur outside the brain or heart, and this represents a large and growing patient population.
Penumbra has developed a suite of thrombectomy and embolization products for use in a range of peripheral vascular conditions:
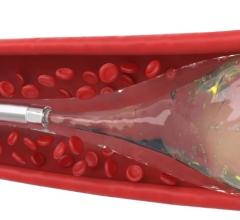
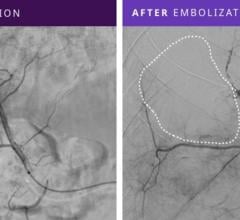
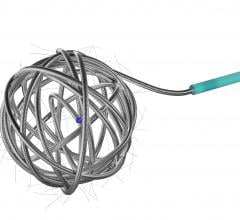
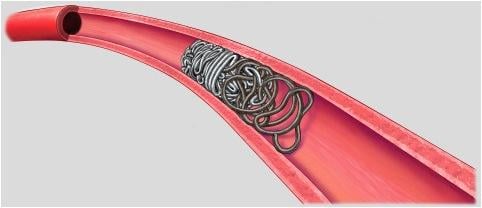
- Penumbra’s embolization platform includes Ruby and POD and the new POD Packing Coil, which is designed to pack very densely behind Ruby and POD to occlude arteries and veins throughout the peripheral vasculature, including aneurysms.
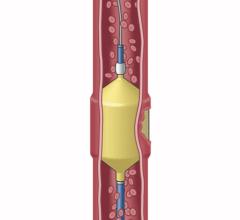
- Penumbra’s next-generation Indigo System is a continuous aspiration thrombectomy device designed to remove fresh, soft emboli and thrombi from the peripheral arteries and veins. The Indigo System includes four catheter sizes (CAT 3, 5, 6 and 8). The aspiration lumen is paired with a proprietary continuous vacuum aspiration pump to evacuate clot effectively and efficiently.
“With the Indigo System and POD, Penumbra has recently introduced products that have had significant impact on the treatment of vascular disease. Indigo represents a significant advancement in the treatment of thrombotic and embolic disease, which until now has had limited treatment options,” said Corey Teigen, M.D., at Sanford Health in Fargo, N.D., who uses Penumbra’s peripheral vascular products. “With the Indigo System, physicians now have the ability to remove limb- and life-threatening clots quickly and efficiently. Likewise the POD, Ruby and now the POD Packing Coil optimize embolization procedures by decreasing procedure time while providing increased control.”
For more information: www.penumbrainc.com


 June 05, 2025
June 05, 2025