February 27, 2008 - Boston Scientific Corp. said the FDA approved three products in its Cardiac Rhythm Management business, including the CONFIENT implantable cardioverter defibrillator (ICD), the LIVIAN cardiac resynchronization therapy defibrillator (CRT-D) and an upgraded LATITUDE Patient Management System.
February 27, 2008 - Corgenix Medical Corp. received notification of European Patent Office (EPO) approval for technology ...
February 27, 2008 - The Center for Connected Health, a division of Partners HealthCare and EMC Corp. will study how EMC ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
February 27, 2008 - diaDexus Inc. said the FDA cleared for marketing a new automated version of its proprietary PLAC ...
February 27, 2008 - ATS Medical Inc. released initial clinical results of stand-alone ablation procedures and an overall ...
February 27, 2008 - CircuLite Inc. released preliminary results of a pilot first-in-man study of four patients who have been implanted with the Synergy Pocket Circulatory Assist Device, a micro implantable blood pump, the size of a AA battery, that can be implanted superficially in a “pacemaker-like” pocket.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The enhanced Xcelera solution. The integrated multimodality solution reportedly provides one access point for all relevant cardiology information, such as cardiovascular ultrasound, CT, MR, nuclear medicine, ECGs and electrophysiology recording signals, as well as long-term storage and enterprise distribution of multimedia studies.
The Precedence SPECT/CT reportedly unites multislice CT with a flexible gamma camera. The CardioMD is a compact, fixed ...
February 26, 2008 - Microsoft Corp. released at HIMSS 2008 its Patient Safety Screening Tool (PSST), a software-based solution designed to help identify potential adverse events that occur during hospitalization, featuring a set of indicators that provide information on potential in-hospital complications following surgeries and medical procedures.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
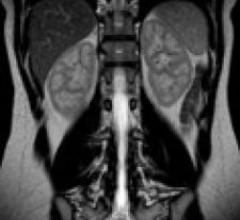
Philips offers the Panorama HFO, Achieva 1.5T A-series, Achieva 3.0T X-series and one of the only 1.5T to 3.0T rampable magnet, the Achieva XR. All Philips MRI systems can take advantage of the complete clinical solutions for cardiac MR with the Elite Clinical Cardiac Solution, said Philips.
Philips' Essence technology is a combination of X-ray tube, detector system and reconstruction design elements designed to improve image quality of CT exams.
February 25, 2008 - FroozHIE from NoMoreClipboard.com reportedly reconciles clinical patient data from disparate sources ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
One of the biggest pain points for medical facilities today is managing increasing volumes of image data. To help ease ...
February 22, 2008 - According to Millennium Research Group’s Global Markets for C-Arms 2008 report, the U.S. market for fixed C-arm systems, comprising units used for cardiology, angiography and neuroradiology procedures, was valued at over $1.4 billion in 2007 and will rise to over $1.8 billion by 2012.
February 21, 2008 - Philips opened the doors to its 50th Ambient Experience suite at Fairview Hospital in Cleveland, OH ...

 February 26, 2008
February 26, 2008